当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Engineering of pH-Responsive NIR Oxazine Assemblies for Evoking Tumor Ferroptosis via Triggering Lysosomal Dysfunction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-02 , DOI: 10.1021/jacs.2c13222 Wei Li 1 , Shulu Yin 1 , Yang Shen 1 , Haiyan Li 1 , Lin Yuan 1 , Xiao-Bing Zhang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-02 , DOI: 10.1021/jacs.2c13222 Wei Li 1 , Shulu Yin 1 , Yang Shen 1 , Haiyan Li 1 , Lin Yuan 1 , Xiao-Bing Zhang 1
Affiliation
|
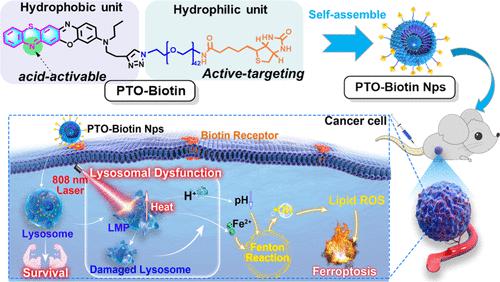
Ferroptosis, a newly discovered form of regulated cell death, is emerging as a promising approach to tumor therapy. However, the spatiotemporal control of cell-intrinsic Fenton chemistry to modulate tumor ferroptosis remains challenging. Here, we report an oxazine-based activatable molecular assembly (PTO-Biotin Nps), which is capable of triggering the lysosomal dysfunction-mediated Fenton pathway with excellent spatiotemporal resolution via near-infrared (NIR) light to evoke ferroptosis. In this system, a pH-responsive NIR photothermal oxazine molecule was designed and functionalized with a tumor-targeting hydrophilic biotin-poly(ethylene glycol) (PEG) chain to engineer well-defined nanostructured assemblies within a single-molecular framework. PTO-Biotin Nps possesses a selective tropism to lysosome accumulation inside tumor cells, accommodated by its enhanced photothermal activity in the acidic microenvironment. Upon NIR light activation, PTO-Biotin Nps promoted lysosomal dysfunction and induced cytosolic acidification and impaired autophagy. More importantly, photoactivation-mediated lysosomal dysfunction via PTO-Biotin Nps was found to markedly enhance cellular Fenton reactions and evoke ferroptosis, thereby improving antitumor efficacy and mitigating systemic side effects. Overall, our study demonstrates that the molecular engineering approach of pH-responsive photothermal oxazine assemblies enables the spatiotemporal modulation of the intrinsic ferroptosis mechanism, offering a novel strategy for the development of metal-free Fenton inducers in antitumor therapy.
中文翻译:

通过触发溶酶体功能障碍诱发肿瘤铁死亡的 pH 响应 NIR 恶嗪组装的分子工程
Ferroptosis 是一种新发现的受调节细胞死亡形式,正在成为一种有前途的肿瘤治疗方法。然而,细胞内芬顿化学调节肿瘤铁死亡的时空控制仍然具有挑战性。在这里,我们报告了一种基于恶嗪的可激活分子组装体 ( PTO-Biotin Nps ),它能够通过近红外 (NIR) 光以出色的时空分辨率触发溶酶体功能障碍介导的 Fenton 通路,从而引发铁死亡。在该系统中,设计了一种 pH 响应性 NIR 光热恶嗪分子,并使用肿瘤靶向亲水性生物素-聚乙二醇 (PEG) 链对其进行功能化,以在单分子框架内设计明确定义的纳米结构组件。PTO-生物素核糖核酸对肿瘤细胞内的溶酶体积累具有选择性趋向性,通过其在酸性微环境中增强的光热活性来适应。在 NIR 光激活后,PTO-Biotin Nps促进溶酶体功能障碍并诱导细胞溶质酸化和自噬受损。更重要的是,光激活介导的溶酶体功能障碍通过PTO-Biotin Nps被发现可显着增强细胞芬顿反应并引起铁死亡,从而提高抗肿瘤疗效并减轻全身副作用。总的来说,我们的研究表明,pH 响应光热恶嗪组装体的分子工程方法能够对内在铁死亡机制进行时空调节,为抗肿瘤治疗中无金属芬顿诱导剂的开发提供了一种新策略。
更新日期:2023-02-02
中文翻译:

通过触发溶酶体功能障碍诱发肿瘤铁死亡的 pH 响应 NIR 恶嗪组装的分子工程
Ferroptosis 是一种新发现的受调节细胞死亡形式,正在成为一种有前途的肿瘤治疗方法。然而,细胞内芬顿化学调节肿瘤铁死亡的时空控制仍然具有挑战性。在这里,我们报告了一种基于恶嗪的可激活分子组装体 ( PTO-Biotin Nps ),它能够通过近红外 (NIR) 光以出色的时空分辨率触发溶酶体功能障碍介导的 Fenton 通路,从而引发铁死亡。在该系统中,设计了一种 pH 响应性 NIR 光热恶嗪分子,并使用肿瘤靶向亲水性生物素-聚乙二醇 (PEG) 链对其进行功能化,以在单分子框架内设计明确定义的纳米结构组件。PTO-生物素核糖核酸对肿瘤细胞内的溶酶体积累具有选择性趋向性,通过其在酸性微环境中增强的光热活性来适应。在 NIR 光激活后,PTO-Biotin Nps促进溶酶体功能障碍并诱导细胞溶质酸化和自噬受损。更重要的是,光激活介导的溶酶体功能障碍通过PTO-Biotin Nps被发现可显着增强细胞芬顿反应并引起铁死亡,从而提高抗肿瘤疗效并减轻全身副作用。总的来说,我们的研究表明,pH 响应光热恶嗪组装体的分子工程方法能够对内在铁死亡机制进行时空调节,为抗肿瘤治疗中无金属芬顿诱导剂的开发提供了一种新策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号