当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improvement of the Ethyl Acetoacetate Preparation Experiment: A Green Chemistry Experiment
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2023-02-01 , DOI: 10.1021/acs.jchemed.2c00718 Da-Zhi Tan 1 , Ming-Ze Li 1 , Wan-nan Xiong 1 , Yi-Xuan Xu 1 , Yang Pan 1 , Wen-Jie Fan 2 , Wen-Feng Jiang 1
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2023-02-01 , DOI: 10.1021/acs.jchemed.2c00718 Da-Zhi Tan 1 , Ming-Ze Li 1 , Wan-nan Xiong 1 , Yi-Xuan Xu 1 , Yang Pan 1 , Wen-Jie Fan 2 , Wen-Feng Jiang 1
Affiliation
|
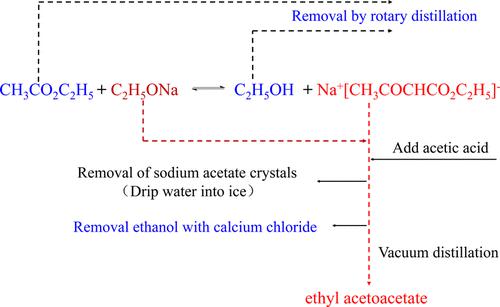
The preparation of ethyl acetoacetate is a distinctive, century-old classic organic chemistry laboratory project. Ethyl acetate and sodium metal (sodium ethoxide) are reacted by Claisen ester condensation to produce the sodium salt of ethyl acetoacetate and then obtain the refined product by acidification as well as washing, drying, and distillation under reduced pressure. This article analyzed the reasons for the low product yield and poor purity of the traditional experimental method and found that ethanol was the key to the final product. The improved experimental method used rotary evaporation and washing with saturated calcium chloride solution to remove ethanol. The new experimental method not only retains the characteristics of the original experiment and improves the product yield, but also adds the interesting phenomenon of pointing water into “ice”, which cultivates students’ learning interest and innovation and achieves a good teaching effect.
中文翻译:

乙酰乙酸乙酯制备实验的改进:绿色化学实验
乙酰乙酸乙酯的制备是一项特色鲜明、具有百年历史的经典有机化学实验室课题。乙酸乙酯与金属钠(乙醇钠)经克莱森酯缩合反应生成乙酰乙酸乙酯的钠盐,再经酸化、洗涤、干燥、减压蒸馏得到精制产品。本文分析了传统实验方法产品收率低、纯度差的原因,发现乙醇是最终产品的关键。改进后的实验方法采用旋转蒸发和饱和氯化钙溶液洗涤去除乙醇。新的实验方法不仅保留了原实验的特点,提高了产品收率,
更新日期:2023-02-01
中文翻译:

乙酰乙酸乙酯制备实验的改进:绿色化学实验
乙酰乙酸乙酯的制备是一项特色鲜明、具有百年历史的经典有机化学实验室课题。乙酸乙酯与金属钠(乙醇钠)经克莱森酯缩合反应生成乙酰乙酸乙酯的钠盐,再经酸化、洗涤、干燥、减压蒸馏得到精制产品。本文分析了传统实验方法产品收率低、纯度差的原因,发现乙醇是最终产品的关键。改进后的实验方法采用旋转蒸发和饱和氯化钙溶液洗涤去除乙醇。新的实验方法不仅保留了原实验的特点,提高了产品收率,































 京公网安备 11010802027423号
京公网安备 11010802027423号