当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cooperative NHC and nickel-catalyzed asymmetric reductive coupling of nitrobenzyl bromides and cyclic ketimines via SET process
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-02-01 , DOI: 10.1039/d2qo01940j
Wen-Tian Zeng 1 , Xiao Han 1 , Gong-Bin Huang 1 , Jiang Weng 1 , Albert S. C. Chan 1 , Gui Lu 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-02-01 , DOI: 10.1039/d2qo01940j
Wen-Tian Zeng 1 , Xiao Han 1 , Gong-Bin Huang 1 , Jiang Weng 1 , Albert S. C. Chan 1 , Gui Lu 1
Affiliation
|
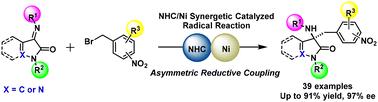
An enantioselective reductive coupling of nitrobenzyl bromides with cyclic ketimines via single-electron-transfer (SET) process was realized via a synergistic combination of N-heterocyclic carbene (NHC) catalysis and nickel catalysis. This protocol could overcome the limitation of homoenolate radical species, providing an important influence on the tight stereocontrol of the nitrobenzyl radical intermediate and offering a direct and facile route to previously inaccessible, enantioenriched 4-substituted 4-aminopyrazolones and 3-substituted 3-aminooxindoles. Moreover, this NHC/metal cooperative catalysis might provide complementary access to new reaction pathways, especially asymmetric radical reactions.
中文翻译:

硝基苄基溴和环酮亚胺通过 SET 过程的协同 NHC 和镍催化不对称还原偶联
通过N-杂环卡宾 (NHC) 催化和镍催化的协同组合,实现了硝基苄基溴与环酮亚胺通过单电子转移 (SET) 过程的对映选择性还原偶联。该协议可以克服 homoenolate 自由基种类的限制,对硝基苄基自由基中间体的严格立体控制产生重要影响,并为以前无法获得的、对映体富集的 4-取代 4-氨基吡唑酮和 3-取代 3-氨基氧吲哚提供直接和简便的途径。此外,这种 NHC/金属协同催化可能为新反应途径提供互补途径,尤其是不对称自由基反应。
更新日期:2023-02-01
中文翻译:

硝基苄基溴和环酮亚胺通过 SET 过程的协同 NHC 和镍催化不对称还原偶联
通过N-杂环卡宾 (NHC) 催化和镍催化的协同组合,实现了硝基苄基溴与环酮亚胺通过单电子转移 (SET) 过程的对映选择性还原偶联。该协议可以克服 homoenolate 自由基种类的限制,对硝基苄基自由基中间体的严格立体控制产生重要影响,并为以前无法获得的、对映体富集的 4-取代 4-氨基吡唑酮和 3-取代 3-氨基氧吲哚提供直接和简便的途径。此外,这种 NHC/金属协同催化可能为新反应途径提供互补途径,尤其是不对称自由基反应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号