Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nature of Catalytic Behavior of Cobalt Oxides for CO2 Hydrogenation
JACS Au ( IF 8.5 ) Pub Date : 2023-02-01 , DOI: 10.1021/jacsau.2c00632
Kailang Li 1 , Xianghong Li 1 , Lulu Li 1 , Xin Chang 1 , Shican Wu 1 , Chengsheng Yang 1 , Xiwen Song 1 , Zhi-Jian Zhao 1 , Jinlong Gong 1, 2, 3, 4
JACS Au ( IF 8.5 ) Pub Date : 2023-02-01 , DOI: 10.1021/jacsau.2c00632
Kailang Li 1 , Xianghong Li 1 , Lulu Li 1 , Xin Chang 1 , Shican Wu 1 , Chengsheng Yang 1 , Xiwen Song 1 , Zhi-Jian Zhao 1 , Jinlong Gong 1, 2, 3, 4
Affiliation
|
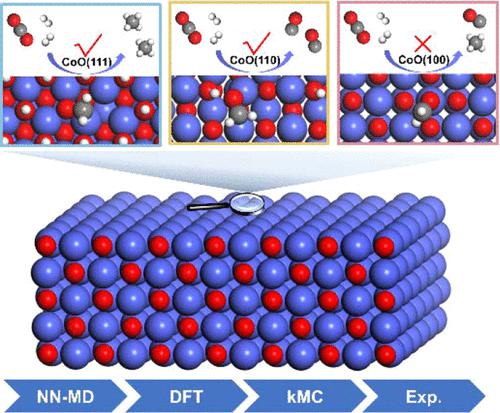
Cobalt oxide (CoOx) catalysts are widely applied in CO2 hydrogenation but suffer from structural evolution during the reaction. This paper describes the complicated structure–performance relationship under reaction conditions. An iterative approach was employed to simulate the reduction process with the help of neural network potential-accelerated molecular dynamics. Based on the reduced models of catalysts, a combined theoretical and experimental study has discovered that CoO(111) provides active sites to break C–O bonds for CH4 production. The analysis of the reaction mechanism indicated that the C–O bond scission of *CH2O species plays a key role in producing CH4. The nature of dissociating C–O bonds is attributed to the stabilization of *O atoms after C–O bond cleavage and the weakening of C–O bond strength by surface-transferred electrons. This work may offer a paradigm to explore the origin of performance over metal oxides in heterogeneous catalysis.
中文翻译:

氧化钴对 CO2 加氢催化行为的性质
氧化钴(CoO x )催化剂广泛应用于CO 2加氢反应,但在反应过程中会发生结构演化。本文描述了反应条件下复杂的结构-性能关系。在神经网络势加速分子动力学的帮助下,采用迭代方法来模拟还原过程。基于催化剂的简化模型,理论和实验相结合的研究发现,CoO(111) 为断裂 C-O 键以生产 CH 4提供了活性位点。反应机理分析表明*CH 2 O物种的C-O键断裂在生成CH 4中起着关键作用。 C-O 键解离的性质归因于 C-O 键断裂后 *O 原子的稳定以及表面转移电子削弱 C-O 键强度。这项工作可能为探索多相催化中金属氧化物的性能起源提供一个范例。
更新日期:2023-02-01
中文翻译:

氧化钴对 CO2 加氢催化行为的性质
氧化钴(CoO x )催化剂广泛应用于CO 2加氢反应,但在反应过程中会发生结构演化。本文描述了反应条件下复杂的结构-性能关系。在神经网络势加速分子动力学的帮助下,采用迭代方法来模拟还原过程。基于催化剂的简化模型,理论和实验相结合的研究发现,CoO(111) 为断裂 C-O 键以生产 CH 4提供了活性位点。反应机理分析表明*CH 2 O物种的C-O键断裂在生成CH 4中起着关键作用。 C-O 键解离的性质归因于 C-O 键断裂后 *O 原子的稳定以及表面转移电子削弱 C-O 键强度。这项工作可能为探索多相催化中金属氧化物的性能起源提供一个范例。

































 京公网安备 11010802027423号
京公网安备 11010802027423号