当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dramatic acceleration by visible light and mechanism of AuPd@ZIF-8-catalyzed ammonia borane methanolysis for efficient hydrogen production
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2023-01-31 , DOI: 10.1039/d2ta08396e Naixin Kang 1 , Ruofan Shen 2 , Baojun Li 2 , Fangyu Fu 3 , Bruno Espuche 4, 5 , Sergio Moya 4 , Lionel Salmon 6 , Jean-Luc Pozzo 1 , Didier Astruc 1
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2023-01-31 , DOI: 10.1039/d2ta08396e Naixin Kang 1 , Ruofan Shen 2 , Baojun Li 2 , Fangyu Fu 3 , Bruno Espuche 4, 5 , Sergio Moya 4 , Lionel Salmon 6 , Jean-Luc Pozzo 1 , Didier Astruc 1
Affiliation
|
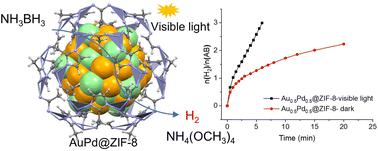
The generation of H2 from materials with a high content of H atoms is attractive for both sustainable energy and convenient hydrogenation. We report that the novel synthesized AuPd@ZIF-8-alloyed nanoparticles (ZIF = zeolitic imidazolate framework) in which the AuPd nanoparticles (NPs) have a size of 2.43 nm and are shown by Brunauer–Emmett–Teller (BET) surface to be encapsulated into ZIF-8. The AuPd and ZIF-8 nanoparticle is an excellent nanocatalyst for the evolution of H2 in the methanolysis reaction of aminoborane (AB) under visible light irradiation. Visible-light-induced acceleration is due to the Au plasmonic excitation provoking hot electron transfer from Au to Pd-substrate ensemble, whereas the reactions catalyzed by monometallic Au@ZIF-8 or Pd@ZIF-8 undergo only few minutes or no acceleration (respectively) under visible light irradiation. Three mol H2 per mol AB are produced in 6 min at 25 °C (TOF: 86.8 mol H2 molatom−1 min−1) with AuPd@ZIF-8 under visible light compared with incomplete H2 formation in 30 min in the dark. A comparison of various heterogeneous supports shows that the ZIF-8 encapsulation of the nanoalloy is by far the best support for this reaction. The large primary kinetic isotope effect (KIE) kH/kD = 3.4 with visible-light irradiation, the high turnover frequency (TOF) under light illumination (3.7 times higher than in the dark), and density functional theory (DFT) calculations confirm the mechanism and illustrate the more difficult O–H oxidative addition on Pd in methanol than in water in this process. This is due to the weaker acidity of methanol compared to that of water. Coupling labeling and tandem reactions with styrene hydrogenation showed that one H atom of H2 formed is provided by AB, while the other one is from methanol. The cleanness of the H2 generated and the recyclability of the NH4B(OMe)4 product render AB methanolysis attractive for low-temperature H2 production devices and tandem reactions.
中文翻译:

可见光显着加速 AuPd@ZIF-8 催化氨硼烷甲醇分解高效制氢的机制
从具有高 H 原子含量的材料中产生 H 2对于可持续能源和方便的氢化具有吸引力。我们报道了新型合成的 AuPd@ZIF-8 合金纳米粒子(ZIF = 沸石咪唑酯骨架),其中 AuPd 纳米粒子 (NPs) 的尺寸为 2.43 nm,Brunauer-Emmett-Teller (BET) 表面显示为封装到 ZIF-8 中。AuPd 和 ZIF-8 纳米粒子是一种出色的 H 2生成纳米催化剂在可见光照射下氨基硼烷(AB)的甲醇分解反应。可见光诱导的加速是由于 Au 等离子体激元激发引起热电子从 Au 转移到 Pd 基底系综,而由单金属 Au@ZIF-8 或 Pd@ZIF-8 催化的反应仅经历几分钟或没有加速(分别)在可见光照射下。与不完全的H 2相比,在可见光下,AuPd@ZIF-8在25 °C 下 6 分钟内每摩尔 AB 产生三摩尔 H 2 (TOF:86.8 摩尔 H 2摩尔原子-1分钟-1 )在黑暗中 30 分钟内形成。各种异质载体的比较表明,纳米合金的 ZIF-8 封装是迄今为止对该反应最好的载体。可见光照射下的大初级动力学同位素效应(KIE)k H / k D = 3.4,光照下的高转换频率(TOF)(比黑暗高3.7倍),以及密度泛函理论( DFT )计算证实了这一机制并说明了在此过程中,甲醇中 Pd 上的 O-H 氧化加成比水中更难。这是因为与水相比,甲醇的酸度较弱。偶联标记和与苯乙烯加氢的串联反应表明,H 2的一个 H 原子形成的是由AB提供的,而另一种来自甲醇。生成的 H 2的清洁度和 NH 4 B(OMe) 4产品的可回收性使得 AB 甲醇分解对于低温 H 2生产装置和串联反应具有吸引力。
更新日期:2023-01-31
中文翻译:

可见光显着加速 AuPd@ZIF-8 催化氨硼烷甲醇分解高效制氢的机制
从具有高 H 原子含量的材料中产生 H 2对于可持续能源和方便的氢化具有吸引力。我们报道了新型合成的 AuPd@ZIF-8 合金纳米粒子(ZIF = 沸石咪唑酯骨架),其中 AuPd 纳米粒子 (NPs) 的尺寸为 2.43 nm,Brunauer-Emmett-Teller (BET) 表面显示为封装到 ZIF-8 中。AuPd 和 ZIF-8 纳米粒子是一种出色的 H 2生成纳米催化剂在可见光照射下氨基硼烷(AB)的甲醇分解反应。可见光诱导的加速是由于 Au 等离子体激元激发引起热电子从 Au 转移到 Pd 基底系综,而由单金属 Au@ZIF-8 或 Pd@ZIF-8 催化的反应仅经历几分钟或没有加速(分别)在可见光照射下。与不完全的H 2相比,在可见光下,AuPd@ZIF-8在25 °C 下 6 分钟内每摩尔 AB 产生三摩尔 H 2 (TOF:86.8 摩尔 H 2摩尔原子-1分钟-1 )在黑暗中 30 分钟内形成。各种异质载体的比较表明,纳米合金的 ZIF-8 封装是迄今为止对该反应最好的载体。可见光照射下的大初级动力学同位素效应(KIE)k H / k D = 3.4,光照下的高转换频率(TOF)(比黑暗高3.7倍),以及密度泛函理论( DFT )计算证实了这一机制并说明了在此过程中,甲醇中 Pd 上的 O-H 氧化加成比水中更难。这是因为与水相比,甲醇的酸度较弱。偶联标记和与苯乙烯加氢的串联反应表明,H 2的一个 H 原子形成的是由AB提供的,而另一种来自甲醇。生成的 H 2的清洁度和 NH 4 B(OMe) 4产品的可回收性使得 AB 甲醇分解对于低温 H 2生产装置和串联反应具有吸引力。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号