Cell Reports ( IF 7.5 ) Pub Date : 2023-01-30 , DOI: 10.1016/j.celrep.2023.112076 Frederick Rehfeld 1 , Jennifer L Eitson 2 , Maikke B Ohlson 2 , Tsung-Cheng Chang 1 , John W Schoggins 2 , Joshua T Mendell 3
|
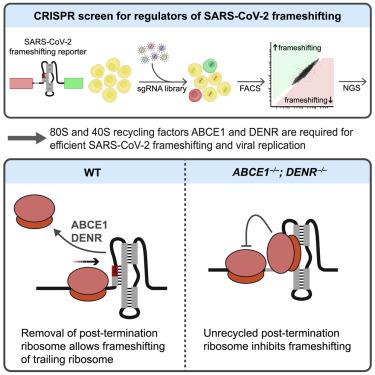
During translation of the genomic RNA of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative virus in the COVID-19 pandemic, host ribosomes undergo programmed ribosomal frameshifting (PRF) at a conserved structural element. Although PRF is essential for coronavirus replication, host factors that regulate this process have not yet been identified. Here we perform genome-wide CRISPR-Cas9 knockout screens to identify regulators of SARS-CoV-2 PRF. These screens reveal that loss of ribosome recycling factors markedly decreases frameshifting efficiency and impairs SARS-CoV-2 viral replication. Mutational studies support a model wherein efficient removal of ribosomal subunits at the ORF1a stop codon is required for frameshifting of trailing ribosomes. This dependency upon ribosome recycling is not observed with other non-pathogenic human betacoronaviruses and is likely due to the unique position of the ORF1a stop codon in the SARS clade of coronaviruses. These findings therefore uncover host factors that support efficient SARS-CoV-2 translation and replication.
中文翻译:

CRISPR 筛选揭示了高效 SARS-CoV-2 程序化核糖体移码和病毒复制对核糖体回收的依赖
在严重急性呼吸综合征冠状病毒 2 (SARS-CoV-2)(COVID-19 大流行中的致病病毒)的基因组 RNA 翻译过程中,宿主核糖体在保守结构元件处经历程序性核糖体移码 (PRF)。尽管 PRF 对于冠状病毒复制至关重要,但调节这一过程的宿主因素尚未确定。在这里,我们进行全基因组 CRISPR-Cas9 敲除筛选,以确定 SARS-CoV-2 PRF 的调节因子。这些筛选表明,核糖体回收因子的丢失会显着降低移码效率并损害 SARS-CoV-2 病毒复制。突变研究支持这样一种模型,其中尾随核糖体的移码需要有效去除 ORF1a 终止密码子处的核糖体亚基。这种对核糖体回收的依赖性在其他非致病性人类 β 冠状病毒中并未观察到,这可能是由于 SARS 冠状病毒进化枝中 ORF1a 终止密码子的独特位置所致。因此,这些发现揭示了支持 SARS-CoV-2 高效翻译和复制的宿主因素。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号