当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ethene Hydroformylation Catalyzed by Rhodium Dispersed with Zinc or Cobalt in Silanol Nests of Dealuminated Zeolite Beta
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-01-30 , DOI: 10.1021/jacs.2c11075 Liang Qi 1, 2, 3 , Sonali Das 4 , Yanfei Zhang 1, 2, 5 , Danna Nozik 1, 2 , Bruce C Gates 4 , Alexis T Bell 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-01-30 , DOI: 10.1021/jacs.2c11075 Liang Qi 1, 2, 3 , Sonali Das 4 , Yanfei Zhang 1, 2, 5 , Danna Nozik 1, 2 , Bruce C Gates 4 , Alexis T Bell 1, 2
Affiliation
|
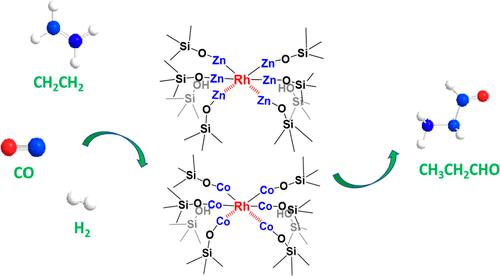
Catalysts for hydroformylation of ethene were prepared by grafting Rh into nests of ≡SiOZn–OH or ≡SiOCo–OH species prepared in dealuminated BEA zeolite. X-ray absorption spectra and infrared spectra of adsorbed CO were used to characterize the dispersion of Rh. The Rh dispersion was found to increase markedly with increasing M/Rh (M = Zn or Co) ratio; further increases in Rh dispersion occurred upon use for ethene hydroformylation catalysis. The turnover frequency for ethene hydroformylation measured for a fixed set of reaction conditions increased with the fraction of atomically dispersed Rh. The ethene hydroformylation activity is 15.5-fold higher for M = Co than for M = Zn, whereas the propanal selectivity is slightly greater for the latter catalyst. The activity of the Co-containing catalyst exceeds that of all previously reported Rh-containing bimetallic catalysts. The rates of ethene hydroformylation and ethene hydrogenation exhibit positive reaction orders in ethene and hydrogen but negative orders in carbon monoxide. In situ IR spectroscopy and the kinetics of the catalytic reactions suggest that ethene hydroformylation is mainly catalyzed by atomically dispersed Rh that is influenced by Rh–M interactions, whereas ethene hydrogenation is mainly catalyzed by Rh nanoclusters. In situ IR spectroscopy also indicates that the ethene hydroformylation is rate limited by formation of propionyl groups and by their hydrogenation, a conclusion supported by the measured H/D kinetic isotope effect. This study presents a novel method for creating highly active Rh-containing bimetallic sites for ethene hydroformylation and provides new insights into the mechanism and kinetics of this process.
中文翻译:

脱铝沸石 β 硅烷醇巢中锌钴分散铑催化乙烯加氢甲酰化反应
通过将 Rh 接枝到在脱铝 BEA 沸石中制备的 ≡SiOZn-OH 或 ≡SiOCo-OH 物种的巢中,制备了用于乙烯加氢甲酰化的催化剂。吸附的 CO 的 X 射线吸收光谱和红外光谱用于表征 Rh 的分散。发现 Rh 分散度随着 M/Rh(M = Zn 或 Co)比率的增加而显着增加;在用于乙烯加氢甲酰化催化时,Rh 分散会进一步增加。在一组固定的反应条件下测量的乙烯加氢甲酰化的周转频率随着原子分散的 Rh 的分数而增加。M = Co 的乙烯加氢甲酰化活性比 M = Zn 高 15.5 倍,而后者催化剂的丙醛选择性略高。含Co催化剂的活性超过了所有先前报道的含Rh双金属催化剂。乙烯加氢甲酰化和乙烯加氢的速率在乙烯和氢气中表现出正反应顺序,而在一氧化碳中表现出负反应顺序。原位红外光谱和催化反应动力学表明,乙烯加氢甲酰化主要由受 Rh-M 相互作用影响的原子分散 Rh 催化,而乙烯加氢主要由 Rh 纳米团簇催化。原位红外光谱还表明,乙烯加氢甲酰化的速率受丙酰基团的形成及其氢化的限制,这一结论得到了测量的 H/D 动力学同位素效应的支持。本研究提出了一种为乙烯加氢甲酰化创造高活性含 Rh 双金属位点的新方法,并为该过程的机理和动力学提供了新的见解。
更新日期:2023-01-30
中文翻译:

脱铝沸石 β 硅烷醇巢中锌钴分散铑催化乙烯加氢甲酰化反应
通过将 Rh 接枝到在脱铝 BEA 沸石中制备的 ≡SiOZn-OH 或 ≡SiOCo-OH 物种的巢中,制备了用于乙烯加氢甲酰化的催化剂。吸附的 CO 的 X 射线吸收光谱和红外光谱用于表征 Rh 的分散。发现 Rh 分散度随着 M/Rh(M = Zn 或 Co)比率的增加而显着增加;在用于乙烯加氢甲酰化催化时,Rh 分散会进一步增加。在一组固定的反应条件下测量的乙烯加氢甲酰化的周转频率随着原子分散的 Rh 的分数而增加。M = Co 的乙烯加氢甲酰化活性比 M = Zn 高 15.5 倍,而后者催化剂的丙醛选择性略高。含Co催化剂的活性超过了所有先前报道的含Rh双金属催化剂。乙烯加氢甲酰化和乙烯加氢的速率在乙烯和氢气中表现出正反应顺序,而在一氧化碳中表现出负反应顺序。原位红外光谱和催化反应动力学表明,乙烯加氢甲酰化主要由受 Rh-M 相互作用影响的原子分散 Rh 催化,而乙烯加氢主要由 Rh 纳米团簇催化。原位红外光谱还表明,乙烯加氢甲酰化的速率受丙酰基团的形成及其氢化的限制,这一结论得到了测量的 H/D 动力学同位素效应的支持。本研究提出了一种为乙烯加氢甲酰化创造高活性含 Rh 双金属位点的新方法,并为该过程的机理和动力学提供了新的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号