Applied Geochemistry ( IF 3.1 ) Pub Date : 2023-01-27 , DOI: 10.1016/j.apgeochem.2023.105587 Chi Zhang , Libin Liu , Hanzhong Jia
|
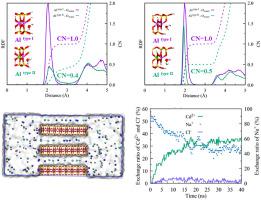
Molecular-level understanding of the exchange processes of heavy metals into the interlayer region of clay minerals help to predict the geochemical cycle of these elements. In this study, we conducted molecular dynamics by using the newly developed CLAYFF-MOH parameters to elucidate the hydration characteristics of edge surfaces. On this basis, we explored the exchange processes of a series of heavy metal cations. Results showed that Mg for Al isomorphous substitution in the secondary outermost positions would reduce the coordination number (CN) of edge Al on both edge surfaces; water films were formed onto the interface owing to H-bonding interaction. Moreover, the cation exchange rates were Pb2+ > Cr3+ > Cd2+ > Ag+ > Ni2+ > Cu2+; Ag+ showed the largest exchange amount, followed by Ni2+, Cd2+, Pb2+, Cu2+, and Cr3+. A small part of Cl− was exchanged along with multivalent cations. The simulations of Ni2+-Cd2+-Pb2+ system indicated that coexistence of divalent cations had no significant effect on the exchange, while the exchange process in Ag+-Cd2+-Cr3+ mixing system was more complicated. Electrostatic interaction was the dominant driving force responsible for exchange. Additionally, Na+ and Ag+ were complexed on the octahedral vacancy, Si–OH, and Al–OH sites. The mobilities of cations decreased with increased ionic valence increased, and overall the diffusion in the interlayer was more constrained than in external solution. This study provides fundamental molecular-level knowledge of the fate of heavy metals in natural environment.
中文翻译:

重金属向蒙脱石交换过程的分子动力学研究:水化边缘表面的表征和动态交换机制
对重金属在粘土矿物层间区域的交换过程的分子水平理解有助于预测这些元素的地球化学循环。在这项研究中,我们通过使用新开发的 CLAYFF-MOH 参数进行分子动力学来阐明边缘表面的水化特性。在此基础上,我们探索了一系列重金属阳离子的交换过程。结果表明,Mg在次要最外层位置同晶取代Al会降低边缘Al在两个边缘表面的配位数(CN);由于氢键相互作用,水膜形成在界面上。阳离子交换率依次为Pb 2+ > Cr 3+ > Cd 2+ > Ag + > Ni2+ > 铜2+ ; Ag +显示出最大的交换量,其次是Ni 2+、Cd 2+、Pb 2+、Cu 2+和Cr 3+。一小部分 Cl -与多价阳离子一起交换。Ni 2+ -Cd 2+ -Pb 2+体系的模拟表明,二价阳离子的共存对交换没有显着影响,而Ag + -Cd 2+ -Cr 3+中的交换过程混合系统更复杂。静电相互作用是负责交换的主要驱动力。此外,Na +和 Ag +在八面体空位、Si–OH 和 Al–OH 位点上络合。阳离子的迁移率随着离子价的增加而降低,总体上层间的扩散比在外部溶液中的扩散更受限制。这项研究提供了关于自然环境中重金属命运的基本分子水平知识。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号