当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deacylative Allylation: Allylic Alkylation via Retro-Claisen Activation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-09-21 , DOI: 10.1021/ja205717f Alexander J Grenning 1 , Jon A Tunge
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-09-21 , DOI: 10.1021/ja205717f Alexander J Grenning 1 , Jon A Tunge
Affiliation
|
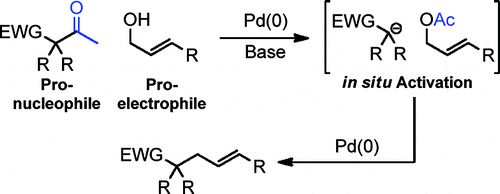
A new method for allylic alkylation of a variety of relatively nonstabilized carbon nucleophiles is described herein. In this process of "deacylative allylation", the coupling partners, an allylic alcohol and a ketone pronucleophile, undergo in situ retro-Claisen activation to generate an allylic acetate and a carbanion. In the presence of palladium, these reactive intermediates undergo catalytic coupling to form a new C-C bond. In comparison to unimolecular decarboxylative allylation, a commonly utilized method for allylation of carbon anions, deacylative allylation is an intermolecular process. Moreover, deacylative allylation allows the direct coupling of readily available allylic alcohols. Lastly, the full utility of deacylative allylation is demonstrated by the rapid construction of a variety 1,6-heptadienes via 3-component couplings.
中文翻译:

脱酰烯丙基化:通过逆克莱森激活进行烯丙基烷基化
本文描述了多种相对不稳定的碳亲核试剂的烯丙基烷基化的新方法。在这个“脱酰烯丙基化”过程中,偶联伙伴(烯丙醇和酮亲核试剂)进行原位逆克莱森活化,生成烯丙乙酸酯和碳负离子。在钯存在下,这些反应性中间体发生催化偶联,形成新的 CC 键。与单分子脱羧烯丙基化(碳阴离子烯丙基化的常用方法)相比,脱酰基烯丙基化是分子间过程。此外,脱酰烯丙基化允许直接偶联容易获得的烯丙醇。最后,通过三元偶联快速构建各种 1,6-庚二烯,证明了脱酰烯丙基化的全部效用。
更新日期:2011-09-21
中文翻译:

脱酰烯丙基化:通过逆克莱森激活进行烯丙基烷基化
本文描述了多种相对不稳定的碳亲核试剂的烯丙基烷基化的新方法。在这个“脱酰烯丙基化”过程中,偶联伙伴(烯丙醇和酮亲核试剂)进行原位逆克莱森活化,生成烯丙乙酸酯和碳负离子。在钯存在下,这些反应性中间体发生催化偶联,形成新的 CC 键。与单分子脱羧烯丙基化(碳阴离子烯丙基化的常用方法)相比,脱酰基烯丙基化是分子间过程。此外,脱酰烯丙基化允许直接偶联容易获得的烯丙醇。最后,通过三元偶联快速构建各种 1,6-庚二烯,证明了脱酰烯丙基化的全部效用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号