当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-Light-Mediated Vicinal Difunctionalization of Activated Alkynes with Boronic Acids: Substrate-Controlled Rapid Access to 3-Alkylated Coumarins and Unsaturated Spirocycles
Organic Letters ( IF 4.9 ) Pub Date : 2023-01-27 , DOI: 10.1021/acs.orglett.2c04333
Sabyasachi Manna 1 , Kandikere Ramaiah Prabhu 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-01-27 , DOI: 10.1021/acs.orglett.2c04333
Sabyasachi Manna 1 , Kandikere Ramaiah Prabhu 1
Affiliation
|
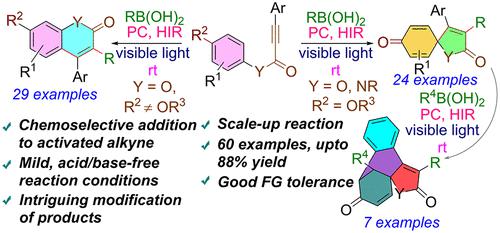
A visible-light-mediated difunctionalization of activated alkynes with boronic acid is unveiled to synthesize 3-alkylated coumarins and unsaturated spiro-lactones. The substituent at the para-position of the aryl ring of aryl alkynoate plays a pivotal role in the selective formation of chain-alkylated coumarins or spirocyclic compounds under mild conditions. The reaction employs a hypervalent iodine reagent and ruthenium photocatalyst. The spiro-lactones thus obtained were subjected to another novel mode of visible-light-mediated radical addition cascade cyclization (RACC) to access various new fused spirocyclic compounds.
中文翻译:

可见光介导的活化炔烃与硼酸的邻位双官能化:底物控制快速获得 3-烷基化香豆素和不饱和螺环
公开了可见光介导的活化炔烃与硼酸的双官能化,以合成 3-烷基化香豆素和不饱和螺内酯。芳基炔酸酯芳基环对位的取代基在温和条件下选择性形成链烷基化香豆素或螺环化合物中起着关键作用。该反应使用高价碘试剂和钌光催化剂。将由此获得的螺内酯进行另一种新型可见光介导的自由基加成级联环化 (RACC) 模式,以获得各种新型稠合螺环化合物。
更新日期:2023-01-27
中文翻译:

可见光介导的活化炔烃与硼酸的邻位双官能化:底物控制快速获得 3-烷基化香豆素和不饱和螺环
公开了可见光介导的活化炔烃与硼酸的双官能化,以合成 3-烷基化香豆素和不饱和螺内酯。芳基炔酸酯芳基环对位的取代基在温和条件下选择性形成链烷基化香豆素或螺环化合物中起着关键作用。该反应使用高价碘试剂和钌光催化剂。将由此获得的螺内酯进行另一种新型可见光介导的自由基加成级联环化 (RACC) 模式,以获得各种新型稠合螺环化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号