Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transferrin Receptor-Mediated Iron Uptake Promotes Colon Tumorigenesis
Advanced Science ( IF 14.3 ) Pub Date : 2023-01-26 , DOI: 10.1002/advs.202207693
Hyeoncheol Kim 1 , Luke B Villareal 1 , Zhaoli Liu 1 , Mohammad Haneef 1 , Daniel M Falcon 1 , David R Martin 2 , Ho-Joon Lee 3 , Michael K Dame 4 , Durga Attili 5 , Ying Chen 6 , James Varani 5 , Jason R Spence 4 , Olga Kovbasnjuk 7 , Justin A Colacino 8 , Costas A Lyssiotis 3 , Henry C Lin 9 , Yatrik M Shah 3 , Xiang Xue 1
Advanced Science ( IF 14.3 ) Pub Date : 2023-01-26 , DOI: 10.1002/advs.202207693
Hyeoncheol Kim 1 , Luke B Villareal 1 , Zhaoli Liu 1 , Mohammad Haneef 1 , Daniel M Falcon 1 , David R Martin 2 , Ho-Joon Lee 3 , Michael K Dame 4 , Durga Attili 5 , Ying Chen 6 , James Varani 5 , Jason R Spence 4 , Olga Kovbasnjuk 7 , Justin A Colacino 8 , Costas A Lyssiotis 3 , Henry C Lin 9 , Yatrik M Shah 3 , Xiang Xue 1
Affiliation
|
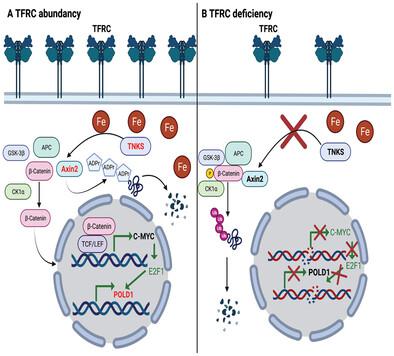
Transferrin receptor (TFRC) is the major mediator for iron entry into a cell. Under excessive iron conditions, TFRC is expected to be reduced to lower iron uptake and toxicity. However, the mechanism whereby TFRC expression is maintained at high levels in iron-enriched cancer cells and the contribution of TFRC to cancer development are enigmatic. Here the work shows TFRC is induced by adenomatous polyposis coli (APC) gene loss-driven β-catenin activation in colorectal cancer, whereas TFRC-mediated intratumoral iron accumulation potentiates β-catenin signaling by directly enhancing the activity of tankyrase. Disruption of TFRC leads to a reduction of colonic iron levels and iron-dependent tankyrase activity, which caused stabilization of axis inhibition protein 2 (AXIN2) and subsequent repression of the β-catenin/c-Myc/E2F Transcription Factor 1/DNA polymerase delta1 (POLD1) axis. POLD1 knockdown, iron chelation, and TFRC disruption increase DNA replication stress, DNA damage response, apoptosis, and reduce colon tumor growth. Importantly, a combination of iron chelators and DNA damaging agents increases DNA damage response and reduces colon tumor cell growth. TFRC-mediated iron import is at the center of a novel feed-forward loop that facilitates colonic epithelial cell survival. This discovery may provide novel strategies for colorectal cancer therapy.
中文翻译:

转铁蛋白受体介导的铁摄取促进结肠肿瘤发生
转铁蛋白受体 (TFRC) 是铁进入细胞的主要介质。在铁过量的条件下,预计会减少 TFRC 以降低铁的摄取和毒性。然而,TFRC 在富铁癌细胞中表达维持在高水平的机制以及 TFRC 对癌症发展的贡献是个谜。这里的工作表明,TFRC 是由结直肠癌中腺瘤性息肉病大肠杆菌 (APC) 基因缺失驱动的 β-catenin 激活诱导的,而 TFRC 介导的瘤内铁积累通过直接增强 tankyrase 的活性来增强 β-catenin 信号传导。TFRC 的破坏导致结肠铁水平和铁依赖性 tankyrase 活性降低,这导致轴抑制蛋白 2 (AXIN2) 的稳定和随后的 β-catenin/c-Myc/E2F 转录因子 1/DNA 聚合酶 delta1 (POLD1) 轴的抑制。POLD1 敲低、铁螯合和 TFRC 破坏会增加 DNA 复制应激、DNA 损伤反应、细胞凋亡,并减少结肠肿瘤生长。重要的是,铁螯合剂和 DNA 损伤剂的组合会增加 DNA 损伤反应并减少结肠肿瘤细胞的生长。TFRC 介导的铁输入处于促进结肠上皮细胞存活的新型前馈环的中心。这一发现可能为结直肠癌治疗提供新的策略。
更新日期:2023-01-26
中文翻译:

转铁蛋白受体介导的铁摄取促进结肠肿瘤发生
转铁蛋白受体 (TFRC) 是铁进入细胞的主要介质。在铁过量的条件下,预计会减少 TFRC 以降低铁的摄取和毒性。然而,TFRC 在富铁癌细胞中表达维持在高水平的机制以及 TFRC 对癌症发展的贡献是个谜。这里的工作表明,TFRC 是由结直肠癌中腺瘤性息肉病大肠杆菌 (APC) 基因缺失驱动的 β-catenin 激活诱导的,而 TFRC 介导的瘤内铁积累通过直接增强 tankyrase 的活性来增强 β-catenin 信号传导。TFRC 的破坏导致结肠铁水平和铁依赖性 tankyrase 活性降低,这导致轴抑制蛋白 2 (AXIN2) 的稳定和随后的 β-catenin/c-Myc/E2F 转录因子 1/DNA 聚合酶 delta1 (POLD1) 轴的抑制。POLD1 敲低、铁螯合和 TFRC 破坏会增加 DNA 复制应激、DNA 损伤反应、细胞凋亡,并减少结肠肿瘤生长。重要的是,铁螯合剂和 DNA 损伤剂的组合会增加 DNA 损伤反应并减少结肠肿瘤细胞的生长。TFRC 介导的铁输入处于促进结肠上皮细胞存活的新型前馈环的中心。这一发现可能为结直肠癌治疗提供新的策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号