当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 1-perfluoroalkyl-3-heteroaryl bicyclo[1.1.1]pentanes via visible light-induced and metal-free perfluoroalkylation of [1.1.1]propellane
Green Chemistry ( IF 9.3 ) Pub Date : 2023-01-27 , DOI: 10.1039/d2gc04683k Boan Yan 1 , Gongcheng Xu 1 , Hang Han 1 , Jun Hong 1 , Wenhao Xu 1 , Deyou Lan 1 , Chuanming Yu 1 , Xinpeng Jiang 1
Green Chemistry ( IF 9.3 ) Pub Date : 2023-01-27 , DOI: 10.1039/d2gc04683k Boan Yan 1 , Gongcheng Xu 1 , Hang Han 1 , Jun Hong 1 , Wenhao Xu 1 , Deyou Lan 1 , Chuanming Yu 1 , Xinpeng Jiang 1
Affiliation
|
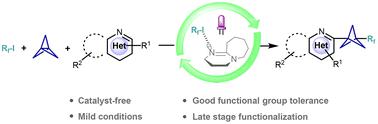
Perfluoroalkyl groups containing bicyclo[1.1.1]pentanes (BCPs) are gaining increasing interest in the pharmaceutical industry and materials science; however, the development of an efficient methodology to construct perfluoroalkylated BCPs remains a challenging task. In this paper, we present a metal-free strategy to synthesize 1-perfluoroalkyl-3-heteroaryl bicyclo[1.1.1]pentanes using an electron donor–acceptor (EDA) complex for the perfluoroalkylation of [1.1.1]propellane. After visible light irradiation, the resulting EDA complexes formed by perfluoroalkyl iodide and DBU lead to the generation of perfluoroalkyl radicals. These then sequentially react with highly strained [1.1.1]propellane and various heterocyclic compounds. Mechanistic studies revealed that this method proceeded via a radical chain reaction. This strategy may provide a versatile and sustainable way to obtain 1-perfluoroalkyl-3-heteroaryl bicyclo[1.1.1]pentanes with good functional group tolerance under mild conditions.
中文翻译:

1-perfluoroalkyl-3-heteroaryl 双环 [1.1.1] 戊烷的合成通过 [1.1.1] propellane 的可见光诱导和无金属全氟烷基化
含有双环 [1.1.1] 戊烷 (BCP) 的全氟烷基在制药行业和材料科学中越来越受到关注;然而,开发构建全氟烷基化 BCP 的有效方法仍然是一项具有挑战性的任务。在本文中,我们提出了一种无金属策略,使用电子供体-受体 (EDA) 复合物合成 1-perfluoroalkyl-3-heteroaryl 双环 [1.1.1] 戊烷,用于 [1.1.1] 螺旋桨的全氟烷基化。在可见光照射后,由全氟烷基碘和 DBU 形成的 EDA 络合物导致全氟烷基自由基的产生。然后它们依次与高度紧张的 [1.1.1] 螺旋桨和各种杂环化合物发生反应。机理研究表明,该方法通过自由基连锁反应。该策略可能提供一种通用且可持续的方法来获得在温和条件下具有良好官能团耐受性的 1-perfluoroalkyl-3-heteroaryl 双环[1.1.1] 戊烷。
更新日期:2023-01-27
中文翻译:

1-perfluoroalkyl-3-heteroaryl 双环 [1.1.1] 戊烷的合成通过 [1.1.1] propellane 的可见光诱导和无金属全氟烷基化
含有双环 [1.1.1] 戊烷 (BCP) 的全氟烷基在制药行业和材料科学中越来越受到关注;然而,开发构建全氟烷基化 BCP 的有效方法仍然是一项具有挑战性的任务。在本文中,我们提出了一种无金属策略,使用电子供体-受体 (EDA) 复合物合成 1-perfluoroalkyl-3-heteroaryl 双环 [1.1.1] 戊烷,用于 [1.1.1] 螺旋桨的全氟烷基化。在可见光照射后,由全氟烷基碘和 DBU 形成的 EDA 络合物导致全氟烷基自由基的产生。然后它们依次与高度紧张的 [1.1.1] 螺旋桨和各种杂环化合物发生反应。机理研究表明,该方法通过自由基连锁反应。该策略可能提供一种通用且可持续的方法来获得在温和条件下具有良好官能团耐受性的 1-perfluoroalkyl-3-heteroaryl 双环[1.1.1] 戊烷。































 京公网安备 11010802027423号
京公网安备 11010802027423号