当前位置:
X-MOL 学术
›
J. Mol. Liq.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A detailed experimental performance of 4-quinolone derivatives as corrosion inhibitors for mild steel in acid media combined with first-principles DFT simulations of bond breaking upon adsorption
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-01-25 , DOI: 10.1016/j.molliq.2023.121299 Caio Machado Fernandes , Amanda R.P. Costa , Mylena C. Leite , Vinicius Martins , Han-Seung Lee , Fernanda da C.S. Boechat , Maria C.B.V. de Souza , Pedro N. Batalha , Hassane Lgaz , Eduardo A. Ponzio
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-01-25 , DOI: 10.1016/j.molliq.2023.121299 Caio Machado Fernandes , Amanda R.P. Costa , Mylena C. Leite , Vinicius Martins , Han-Seung Lee , Fernanda da C.S. Boechat , Maria C.B.V. de Souza , Pedro N. Batalha , Hassane Lgaz , Eduardo A. Ponzio
|
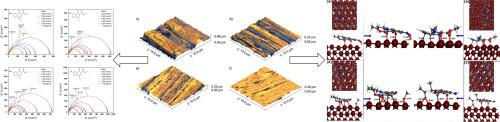
Four 4-oxo-1,4-dihydroquinoline-3-carboxylate derivatives were synthesized through the Gould-Jacobs method and evaluated as corrosion inhibitors for 1020 mild steel in 1 mol/L hydrochloric acid. Gravimetric experiments showed that those organic molecules present 84–94 % anticorrosive efficiency at 2.00 mmol/L (298 K). At higher temperatures (318 and 338 K), those values go up to 97.3 % for the methoxy-substituted compound. Electrochemical measurements depicted that the charge-transfer mechanism controlled the corrosive and inhibitive processes and that the presence of the four organic substances in the electrolyte enhanced the polarization resistance and significantly diminished the corrosion density current, acting by adsorption on the metal surface. Polarization curves confirmed that they all are mixed-type corrosion inhibitors. Atomic Force Microscopy illustrated the topography of the metallic surface and suggested to the formation of a protective layer. Atomistic simulations by first-principles Density Functional Theory revealed the formation of covalent bonds between quinolone molecules and the iron surface, with MODC and AODC having the stronger negative interaction energy values compared to NODC and CODC compounds. Electronic analysis of the adsorption geometries of molecules at Fe(1
中文翻译:

4-喹诺酮类衍生物作为低碳钢在酸性介质中缓蚀剂的详细实验性能,结合吸附时键断裂的第一性原理 DFT 模拟
通过 Gould-Jacobs 方法合成了 4 种 4-氧代-1,4-二氢喹啉-3-羧酸酯衍生物,并评价其作为 1020 低碳钢在 1 mol/L 盐酸中的缓蚀剂。重量实验表明,这些有机分子在 2.00 mmol/L (298 K) 时具有 84-94% 的防腐效率。在较高温度(318 和 338 K)下,甲氧基取代化合物的这些值高达 97.3%。电化学测量表明,电荷转移机制控制腐蚀和抑制过程,并且电解质中四种有机物质的存在增强了极化电阻并显着降低了腐蚀密度电流,通过吸附在金属表面起作用。极化曲线证实它们都是混合型缓蚀剂。原子力显微镜说明了金属表面的形貌,并建议形成保护层。第一性原理密度泛函理论的原子模拟揭示了喹诺酮分子与铁表面之间形成共价键,与 NODC 和 CODC 化合物相比,MODC 和 AODC 具有更强的负相互作用能值。对 Fe(110) 处分子吸附几何形状的电子分析表明,化学配位是吸附时强电荷转移和电荷重排的结果。
更新日期:2023-01-25
中文翻译:

4-喹诺酮类衍生物作为低碳钢在酸性介质中缓蚀剂的详细实验性能,结合吸附时键断裂的第一性原理 DFT 模拟
通过 Gould-Jacobs 方法合成了 4 种 4-氧代-1,4-二氢喹啉-3-羧酸酯衍生物,并评价其作为 1020 低碳钢在 1 mol/L 盐酸中的缓蚀剂。重量实验表明,这些有机分子在 2.00 mmol/L (298 K) 时具有 84-94% 的防腐效率。在较高温度(318 和 338 K)下,甲氧基取代化合物的这些值高达 97.3%。电化学测量表明,电荷转移机制控制腐蚀和抑制过程,并且电解质中四种有机物质的存在增强了极化电阻并显着降低了腐蚀密度电流,通过吸附在金属表面起作用。极化曲线证实它们都是混合型缓蚀剂。原子力显微镜说明了金属表面的形貌,并建议形成保护层。第一性原理密度泛函理论的原子模拟揭示了喹诺酮分子与铁表面之间形成共价键,与 NODC 和 CODC 化合物相比,MODC 和 AODC 具有更强的负相互作用能值。对 Fe(110) 处分子吸附几何形状的电子分析表明,化学配位是吸附时强电荷转移和电荷重排的结果。






























 京公网安备 11010802027423号
京公网安备 11010802027423号