Synthesis ( IF 2.2 ) Pub Date : 2023-01-25 , DOI: 10.1055/a-2004-1093 Emily Kaylin Burke 1 , Erin Norah Welsh 1 , Katherine Nancy Robertson 2 , Alexander W. H. Speed 1
|
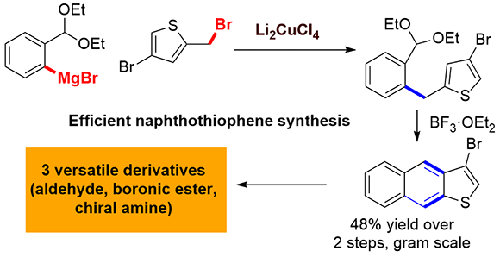
Naphtho[2,3-b]thiophene is a linear sulfur-containing polycyclic aromatic hydrocarbon. Naphtho[2,3-b]thiophene and its derivatives are commonly accessed by a Bradsher cyclization. Synthesis of the Bradsher cyclization substrate typically requires harsh conditions, including several oxidation state changes. Here, we report an improved, multigram synthesis of 3-bromonaphtho[2,3-b]thiophene, exploiting a copper-catalyzed cross-coupling to prepare the Bradsher substrate in three steps from commercial materials while minimizing redox reactions. In this work, the 3-bromonaphthothiophene is further functionalized via lithium–halogen exchange, with the key finding being a specific order of addition in lithiation is required to avoid undesired rearrangement reactions. Transformation to a versatile set of derivatives, including a naphthothiophene-containing chiral amine, is illustrated.
中文翻译:

3-溴萘并[2,3-b]噻吩的高效合成与功能化
萘并[2,3 - b ]噻吩是一种直链含硫多环芳烃。萘并[2,3- b ]噻吩及其衍生物通常通过 Bradsher 环化获得。Bradsher 环化底物的合成通常需要苛刻的条件,包括几个氧化态变化。在这里,我们报告了 3-bromonaphtho[2,3- b的改进的多字母合成]噻吩,利用铜催化的交叉偶联从商业材料分三步制备 Bradsher 底物,同时最大限度地减少氧化还原反应。在这项工作中,3-溴萘并噻吩通过锂-卤素交换进一步功能化,关键发现是锂化中需要特定的添加顺序以避免不希望的重排反应。说明了转化为一组通用的衍生物,包括含有萘并噻吩的手性胺。






























 京公网安备 11010802027423号
京公网安备 11010802027423号