当前位置:
X-MOL 学术
›
J. Mater. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Donor engineering of diphenylamine-substituted tris(2,4,6-trichlorophenyl)methyl radicals for controlling the intramolecular charge transfer and near-infrared photothermal conversion
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2023-01-24 , DOI: 10.1039/d2tc04508g Chuan Yan 1 , Jing Fang 1 , Jiangyu Zhu 2 , Weinan Chen 1 , Min Wang 1 , Kai Chi 2 , Xuefeng Lu 2 , Gang Zhou 1 , Yunqi Liu 2
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2023-01-24 , DOI: 10.1039/d2tc04508g Chuan Yan 1 , Jing Fang 1 , Jiangyu Zhu 2 , Weinan Chen 1 , Min Wang 1 , Kai Chi 2 , Xuefeng Lu 2 , Gang Zhou 1 , Yunqi Liu 2
Affiliation
|
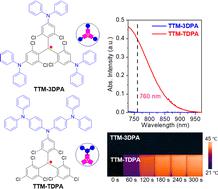
Donor engineering plays an important role in tuning the intramolecular charge transfer (ICT) interactions of donor–acceptor (D–A) molecules and the related performance in the further applications. In comparison to the complicated and time-consuming molecular skeleton exploitation, optimization of the donor number and the substitution position is more facile and efficient. Herein, different quantities of diphenylamine (DPA) units are incorporated into the tris(2,4,6-trichlorophenyl)methyl (TTM) radicals from various directions. The effects of the donor number and the substitution position on the ICT interactions and the photothermal conversion performance are investigated. It is found that TTM-DPA (644 nm), TTM-2DPA (650 nm), and TTM-3DPA (637 nm) with various DPA units attached on different arms of the TTM core exhibit similar absorption maxima and high photoluminescence (PL) quantum yields (over 56%) in cyclohexane solutions. However, when multi-DPA units are introduced on the unilateral arm of the TTM unit, the absorption maximum red shifts to 675 nm for TTM-BDPA and 717 nm for TTM-TDPA. Meanwhile, no obvious PL can be detected using a spectrometer. The near-infrared (NIR) absorbance and the nonradiative transition of the TTM radicals contribute to their photothermal conversion. Consequently, remarkable photothermal conversion efficiencies of 41% and 50% are achieved for TTM-BDPA and TTM-TDPA, respectively. Our study provides an alternative design rule for controlling the ICT effect and shows that organic radicals have potential for application in photothermal therapy in the near future.
中文翻译:

二苯胺取代的三(2,4,6-三氯苯基)甲基自由基的供体工程控制分子内电荷转移和近红外光热转化
供体工程在调节供体-受体 (D-A) 分子的分子内电荷转移 (ICT) 相互作用以及进一步应用中的相关性能方面起着重要作用。与复杂且耗时的分子骨架开发相比,供体数量和取代位置的优化更加容易和高效。在此,不同数量的二苯胺 (DPA) 单元从不同方向并入三 (2,4,6-三氯苯基) 甲基 (TTM) 基团。研究了供体数量和取代位置对 ICT 相互作用和光热转换性能的影响。发现TTM-DPA (644 nm)、TTM-2DPA (650 nm) 和TTM-3DPA(637 nm) 的不同 DPA 单元连接在 TTM 核心的不同臂上,在环己烷溶液中表现出相似的吸收最大值和高光致发光 (PL) 量子产率(超过 56%)。然而,当在 TTM 单元的单侧臂上引入多 DPA 单元时,TTM-BDPA的最大吸收红移到 675 nm, TTM-TDPA的最大吸收红移到717 nm 。同时,使用光谱仪无法检测到明显的 PL。TTM 自由基的近红外 (NIR) 吸收和非辐射跃迁有助于它们的光热转换。因此, TTM-BDPA和TTM-TDPA实现了 41% 和 50% 的显着光热转换效率, 分别。我们的研究为控制 ICT 效应提供了一种替代设计规则,并表明有机自由基在不久的将来具有应用于光热疗法的潜力。
更新日期:2023-01-24
中文翻译:

二苯胺取代的三(2,4,6-三氯苯基)甲基自由基的供体工程控制分子内电荷转移和近红外光热转化
供体工程在调节供体-受体 (D-A) 分子的分子内电荷转移 (ICT) 相互作用以及进一步应用中的相关性能方面起着重要作用。与复杂且耗时的分子骨架开发相比,供体数量和取代位置的优化更加容易和高效。在此,不同数量的二苯胺 (DPA) 单元从不同方向并入三 (2,4,6-三氯苯基) 甲基 (TTM) 基团。研究了供体数量和取代位置对 ICT 相互作用和光热转换性能的影响。发现TTM-DPA (644 nm)、TTM-2DPA (650 nm) 和TTM-3DPA(637 nm) 的不同 DPA 单元连接在 TTM 核心的不同臂上,在环己烷溶液中表现出相似的吸收最大值和高光致发光 (PL) 量子产率(超过 56%)。然而,当在 TTM 单元的单侧臂上引入多 DPA 单元时,TTM-BDPA的最大吸收红移到 675 nm, TTM-TDPA的最大吸收红移到717 nm 。同时,使用光谱仪无法检测到明显的 PL。TTM 自由基的近红外 (NIR) 吸收和非辐射跃迁有助于它们的光热转换。因此, TTM-BDPA和TTM-TDPA实现了 41% 和 50% 的显着光热转换效率, 分别。我们的研究为控制 ICT 效应提供了一种替代设计规则,并表明有机自由基在不久的将来具有应用于光热疗法的潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号