当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactivity of Alkynyl Phosphines with Lewis Acids for the Synthesis of Allenic Phosphonium Borate Zwitterions
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.1 ) Pub Date : 2023-01-13 , DOI: 10.1002/zaac.202200383 Lucas Christian Torres 1 , Amandeep Brar 2 , Jesse LeBlanc 2 , Clive Boateng Ameyaw 2 , Christopher Blain Caputo 1
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.1 ) Pub Date : 2023-01-13 , DOI: 10.1002/zaac.202200383 Lucas Christian Torres 1 , Amandeep Brar 2 , Jesse LeBlanc 2 , Clive Boateng Ameyaw 2 , Christopher Blain Caputo 1
Affiliation
|
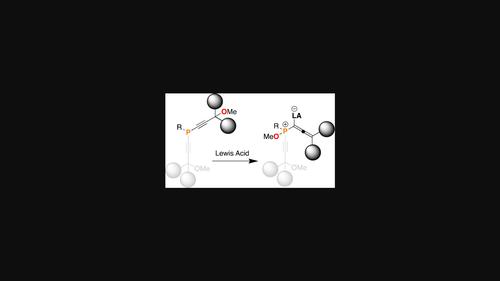
The reactivity of alkynylphosphines with Lewis acidic boranes has been well established in recent years thanks to the advent of frustrated Lewis pairs (FLPs), often leading to a 1,1-carboboration reaction and the formation of an intramolecular phosphinoborane FLP. Our group recently discovered that introduction of an ionizable group in the γ-position of an alkynylphosphine result in an unexpected rearrangement reaction to yield zwitterionic allenic-phosphonium borate products which are produced via a transient 3-coordinate phosphonium allenylidene. Herein, we describe how substituent effects on the γ-carbon impact this reactivity and explore how bis(alkynyl)phosphines behave under similar reaction conditions.
中文翻译:

炔基膦与路易斯酸的反应性用于合成丙二烯硼酸盐两性离子
近年来,由于受挫路易斯对 (FLP) 的出现,炔基膦与路易斯酸性硼烷的反应性得到了很好的证实,通常会导致 1,1-碳硼化反应和分子内膦基硼烷 FLP 的形成。我们小组最近发现,在炔基膦的 γ 位引入可电离基团会导致意想不到的重排反应,从而产生两性离子丙二烯-硼酸盐产物,这些产物是通过瞬时 3 配位亚丙基膦产生的。在此,我们描述了 γ- 碳的取代基效应如何影响这种反应性,并探讨了双(炔基)膦在类似反应条件下的行为方式。
更新日期:2023-01-13
中文翻译:

炔基膦与路易斯酸的反应性用于合成丙二烯硼酸盐两性离子
近年来,由于受挫路易斯对 (FLP) 的出现,炔基膦与路易斯酸性硼烷的反应性得到了很好的证实,通常会导致 1,1-碳硼化反应和分子内膦基硼烷 FLP 的形成。我们小组最近发现,在炔基膦的 γ 位引入可电离基团会导致意想不到的重排反应,从而产生两性离子丙二烯-硼酸盐产物,这些产物是通过瞬时 3 配位亚丙基膦产生的。在此,我们描述了 γ- 碳的取代基效应如何影响这种反应性,并探讨了双(炔基)膦在类似反应条件下的行为方式。






























 京公网安备 11010802027423号
京公网安备 11010802027423号