Chinese Journal of Chemical Engineering Pub Date : 2023-01-20 , DOI: 10.1016/j.cjche.2023.01.006 Dongdong Hu , Yinglei Wang , Chuan Xiao , Yifei Hu , Zhiyong Zhou , Zhongqi Ren
|
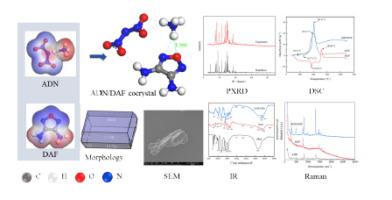
Ammonium dinitramide (ADN) is a promising oxidizer with high energy characteristic, which is a relatively new environmentally friendly oxidizer without halogens and carbon elements. However, ADN has high hygroscopicity when exposed to high humidity air, restricting its applications on the solid propellants. In this paper, a novel energetic cocrystal composed of ammonium dinitramide and 3,4-diaminofurazan (DAF) was proposed and successfully synthesized by antisolvent crystallization method, and the properties of the cocrystal were systematically investigated by analytical characterization and theoretical simulation calculations. The formation of the cocrystal was confirmed by powder X-ray diffraction, differential scanning calorimetry, scanning electron microscopy, infrared spectroscopy and Raman spectroscopy, indicating that the synthesized product was a cocrystal. Through theoretical studies, the ADN/DAF cocrystal structure was predicted, and the powder X-ray diffraction, morphology, water sorption capacity of ADN/DAF cocrystal were calculated, which was consistent with experimental phenomena. The results showed that newly prepared cocrystal of ADN/DAF had lower hygroscopicity compared to pure ADN, and the water sorption capacity was reduced from 15.35% to 7.90%. This may be due to the formation of N H⋯O medium-strength hydrogen bonds between the ammonium ion of ADN and the O atom of DAF in the cocrystal, which prevents the binding of water molecules in the air and ammonium ions and reduces the probability of ADN binding to water molecules, leading to the reduction of cocrystal hygroscopicity. The newly prepared energetic cocrystal can provide theoretical and technical guidance for the study of the anti-hygroscopicity of ADN and advance the practical application of ADN.
H⋯O medium-strength hydrogen bonds between the ammonium ion of ADN and the O atom of DAF in the cocrystal, which prevents the binding of water molecules in the air and ammonium ions and reduces the probability of ADN binding to water molecules, leading to the reduction of cocrystal hygroscopicity. The newly prepared energetic cocrystal can provide theoretical and technical guidance for the study of the anti-hygroscopicity of ADN and advance the practical application of ADN.
中文翻译:

二硝酰胺铵与3,4-二氨基呋喃共晶调节吸湿性的研究
二硝酰胺铵(ADN)是一种很有前景的高能氧化剂,是一种较新型的不含卤素和碳元素的环保氧化剂。然而,ADN暴露在高湿度空气中时具有较高的吸湿性,限制了其在固体推进剂上的应用。本文提出了一种由二硝酰胺铵和3,4-二氨基呋喃赞(DAF)组成的新型含能共晶,并通过反溶剂结晶法成功合成,并通过分析表征和理论模拟计算系统地研究了该共晶的性能。通过粉末X射线衍射、差示扫描量热法、扫描电子显微镜、红外光谱和拉曼光谱证实了共晶的形成,表明合成产物为共晶。通过理论研究,预测了ADN/DAF共晶的结构,并计算了ADN/DAF共晶的粉末X射线衍射、形貌、吸水能力,与实验现象一致。结果表明,新制备的ADN/DAF共晶较纯ADN具有较低的吸湿性,吸水量由15.35%降低至7.90%。这可能是由于N的形成 吸水量由15.35%降低至7.90%。这可能是由于N的形成 吸水量由15.35%降低至7.90%。这可能是由于N的形成 共晶中ADN的铵离子与DAF的O原子之间形成H⋯O中等强度的氢键,阻止了空气中的水分子与铵离子的结合,降低了ADN与水分子结合的概率,从而导致共晶吸湿性的降低。新制备的含能共晶可为ADN的抗吸湿性研究提供理论和技术指导,推进ADN的实际应用。
共晶中ADN的铵离子与DAF的O原子之间形成H⋯O中等强度的氢键,阻止了空气中的水分子与铵离子的结合,降低了ADN与水分子结合的概率,从而导致共晶吸湿性的降低。新制备的含能共晶可为ADN的抗吸湿性研究提供理论和技术指导,推进ADN的实际应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号