Journal of Catalysis ( IF 6.5 ) Pub Date : 2023-01-23 , DOI: 10.1016/j.jcat.2023.01.022 Xiao-Chao Chen , Tian Lan , Jian Zhu , Sibin Ying , Guang-Hui Shi , Kai-Chun Zhao , Lin Guo , Yong Lu , Ye Liu
|
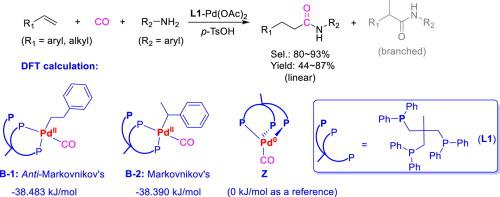
The tripodal phosphine [L1, 1,1,1-tris(diphenylphosphino-methyl)ethane] was applied to control the activity, regioselectivity, and stability of the involved Pd-catalyst in anti-Markovnikov hydroaminocarbonylation of alkenes with aromatic amines for the synthesis of linear-amides. Due to the unique characters of L1 in terms of the steric bulkiness, the switching-coordination mode from tripodal phosphine to bidental one, and the robustness against moisture and oxygen, L1 conferred to Pd-catalyst with the high activity, good regioselectivity, and excellent stability for this carbonylation, leading to 44 ∼ 87 % yields of the target amides with L/B of greater than 80/20. The in situ high-pressure FT-IR analysis and the DFT calculations verified that L1 not only promoted the formation and stability of PdII-H active species (ν ca. 1932 cm−1) but also facilitated the anti-Markovnikov’s addition of alkenes with PdII-H bond due to the favorable energy level of linear intermediate (B-1). In addition, since L1 can coordinate to Pd(II)-center as a bidental chelator as fulfill catalysis and then coordinate to Pd(0)-center as an tripodal pincer, Pd-catalyst was protected timely by L1 against metal-deposition (Pd0-black precipitation) to guarantee its stability for recycling uses for 5 runs without activity loss.
中文翻译:

高效稳定的三脚磷化氢控制钯催化剂用于烯烃与芳香胺的反马尔可夫尼科夫氢氨基羰基化
三足膦 [ L1 , 1,1,1-tris(diphenylphosphino-methyl)ethane] 用于控制所涉及的 Pd 催化剂在烯烃与芳香胺的反马尔可夫尼科夫氢氨基羰基化反应中的活性、区域选择性和稳定性,以合成合成线性酰胺。由于L1在空间体积、从三齿膦到双齿膦的转换配位模式以及对水分和氧气的稳健性方面的独特特性, L1赋予Pd催化剂高活性、良好的区域选择性和优异的性能这种羰基化的稳定性,导致目标酰胺的产率为 44 ∼ 87%,L/B 大于 80/20。就地_高压FT-IR分析和DFT计算证实L1不仅促进了Pd II -H活性物质(ν ca. 1932 cm -1 )的形成和稳定性,而且促进了反马尔可夫尼科夫烯烃与Pd II的加成-H 键由于线性中间体 ( B-1 )的有利能级。此外,由于L1可以作为双齿螯合剂与Pd(II)中心配位作为完成催化,然后作为三足钳与Pd(0)中心配位,L1及时保护Pd催化剂免受金属沉积(Pd 0-黑色沉淀)以保证其循环使用的稳定性5次运行无活性损失。































 京公网安备 11010802027423号
京公网安备 11010802027423号