Chem ( IF 19.1 ) Pub Date : 2023-01-20 , DOI: 10.1016/j.chempr.2022.12.008 Yuping Pan , Yingying Li , Chung-Li Dong , Yu-Cheng Huang , Jingcheng Wu , Jianqiao Shi , Yuxuan Lu , Ming Yang , Shuangyin Wang , Yuqin Zou
|
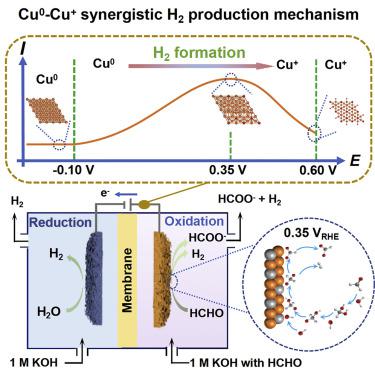
The ultra-low potential oxidation of aldehydes is a conspicuous replacement for oxygen evolution reaction due to its efficiency in lowering the energy required for hydrogen production and producing H2 on dual sides of the electrolyzer. However, the activity origin of catalysts and the reaction mechanism remains unclear. Herein, a formaldehyde oxidation reaction (FOR) with high current density (165 mA cm−2 at 0.35 VRHE) is realized on partially reduced CuO on Cu foam (CuxO@CF) electrocatalysts. In situ characterizations and density functional theory calculations identified and investigated the existence of Cu0 and Cu+ and the synergistic effect for FOR and corroborate the reaction pathway. The results reveal that Cu0 can facilitate the reaction energy of HOCH2O∗ + HO∗, and Cu+ is more favorable for C–H bond cleavage. Meanwhile, H atoms in the produced H2 are verified to be entirely from HCHO. This work provides theoretical guidance for designing Cu-based electrocatalysts for FOR.
中文翻译:

揭示多价铜物种促进甲醛氧化阳极制氢的协同作用
醛类的超低电位氧化是析氧反应的显着替代,因为它可以有效降低电解槽两侧产生氢气和产生 H 2所需的能量。然而,催化剂的活性来源和反应机理仍不清楚。在此,在泡沫铜 (Cu x O@CF) 电催化剂上实现了部分还原的 CuO 的高电流密度(165 mA cm -2在 0.35 V RHE )的甲醛氧化反应(FOR) 。原位表征和密度泛函理论计算确定并研究了 Cu 0和 Cu +的存在以及 FOR 的协同作用,证实了反应途径。结果表明,Cu 0可以促进HOCH 2 O ∗ + HO ∗的反应能,Cu +更有利于C-H键的断裂。同时,证实生成的H 2中的H原子完全来自HCHO。这项工作为设计用于 FOR 的铜基电催化剂提供了理论指导。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号