当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Safety, Biodistribution, and Dosimetry Study of Meplazumab, a Potential COVID-19 Therapeutic Drug, with 131I-Labeling and SPECT Imaging
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2023-01-20 , DOI: 10.1021/acs.molpharmaceut.2c00954 Jiajun Ye 1 , Weidong Yang 1 , Zhaojuan Xie 1 , Yuhao Yan 1 , Guoquan Li 1 , Guiyu Li 1 , Xiang Li 1 , Wenhui Ma 1 , Fei Kang 1 , Mingru Zhang 1 , Jing Wang 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2023-01-20 , DOI: 10.1021/acs.molpharmaceut.2c00954 Jiajun Ye 1 , Weidong Yang 1 , Zhaojuan Xie 1 , Yuhao Yan 1 , Guoquan Li 1 , Guiyu Li 1 , Xiang Li 1 , Wenhui Ma 1 , Fei Kang 1 , Mingru Zhang 1 , Jing Wang 1
Affiliation
|
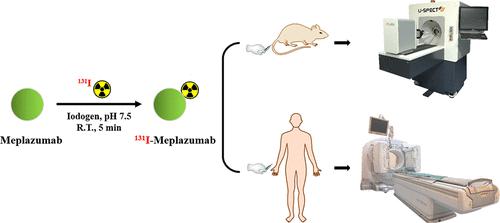
Coronavirus disease 2019 (COVID-19) is a serious threat to public health and is in urgent need of specific drugs. Meplazumab, a humanized monoclonal antibody targeting CD147, was confirmed to competitively block the binding between the spike of syndrome coronavirus 2 (SARS-CoV-2) and CD147, making meplazumab a promising candidate drug for COVID-19. In this study, biodistribution and dosimetry of 131I-labeled meplazumab were performed to further evaluate its potential as a therapeutic drug for COVID-19. 131I-meplazumab was both safe and tolerant in mice and healthy volunteers. A biodistribution study was performed in normal mice, and blood samples were used for pharmacokinetic analysis. Three healthy volunteers were included and subjected to single-photon-emission computed tomography (SPECT) imaging of 131I-meplazumab within 2 weeks. The distribution in mice and humans was consistent with the in vivo distribution of CD147. Biodistribution and SPECT imaging results exhibited that the liver was the organ with the highest uptake for both mice and humans. Deiodination of 131I-meplazumab can be observed in vivo, and taking Lugol’s solution can protect the thyroid gland effectively. The pharmacokinetic characteristics of 131I-meplazumab in mice and humans best fit the two-compartment model. The clearance half-life (T1/2β) in mice and humans was 117.4 and 223.5 h, respectively. The results indicated that its pharmacokinetic properties in vivo were ideal. The effective dose calculated from healthy volunteers was 0.811 ± 0.260 mSv·MBq–1, which was twice the value calculated from mice. It was safe and feasible to perform human clinical imaging experiments using a diagnostic dose of 131I-meplazumab after thyroid closure by Lugol’s solution. This study will provide more experimental basis for advancing the clinical translation of meplazumab and will be valuable in evaluating therapeutic interventions for patients with COVID-19, as well as providing a reference for clinical translation studies of other antibody drugs.
中文翻译:

Meplazumab(一种潜在的 COVID-19 治疗药物)的安全性、生物分布和剂量测定研究,采用 131I 标记和 SPECT 成像
2019年冠状病毒病(COVID-19)对公众健康构成严重威胁,急需特效药物。Meplazumab 是一种靶向 CD147 的人源化单克隆抗体,已被证实能够竞争性阻断刺突综合征冠状病毒 2 (SARS-CoV-2) 与 CD147 之间的结合,使 meplazumab 成为治疗 COVID-19 的有前途的候选药物。在这项研究中,对131 I 标记的 meplazumab 进行了生物分布和剂量测定,以进一步评估其作为 COVID-19 治疗药物的潜力。131 I-meplazumab 在小鼠和健康志愿者中既安全又具有耐受性。在正常小鼠中进行生物分布研究,并使用血液样本进行药代动力学分析。三名健康志愿者被纳入研究,并在 2 周内接受了131 I-meplazumab的单光子发射计算机断层扫描 (SPECT) 成像。小鼠和人类中的分布与CD147的体内分布一致。生物分布和 SPECT 成像结果表明,肝脏是小鼠和人类吸收最高的器官。体内可观察到131 I-meplazumab的脱碘作用,服用 Lugol 溶液可有效保护甲状腺。131 I-meplazumab 在小鼠和人类中的药代动力学特征最符合两室模型。小鼠和人类的清除半衰期(T 1/2 β)分别为 117.4 和 223.5 小时。结果表明其体内药代动力学特性较为理想。健康志愿者计算出的有效剂量为0.811±0.260 mSv·MBq –1,是小鼠计算值的两倍。卢戈氏溶液封堵甲状腺后,使用诊断剂量的131 I-meplazumab进行人体临床影像实验是安全可行的。该研究将为推进meplazumab的临床转化提供更多的实验依据,对于评估COVID-19患者的治疗干预措施具有重要价值,也为其他抗体药物的临床转化研究提供参考。
更新日期:2023-01-20
中文翻译:

Meplazumab(一种潜在的 COVID-19 治疗药物)的安全性、生物分布和剂量测定研究,采用 131I 标记和 SPECT 成像
2019年冠状病毒病(COVID-19)对公众健康构成严重威胁,急需特效药物。Meplazumab 是一种靶向 CD147 的人源化单克隆抗体,已被证实能够竞争性阻断刺突综合征冠状病毒 2 (SARS-CoV-2) 与 CD147 之间的结合,使 meplazumab 成为治疗 COVID-19 的有前途的候选药物。在这项研究中,对131 I 标记的 meplazumab 进行了生物分布和剂量测定,以进一步评估其作为 COVID-19 治疗药物的潜力。131 I-meplazumab 在小鼠和健康志愿者中既安全又具有耐受性。在正常小鼠中进行生物分布研究,并使用血液样本进行药代动力学分析。三名健康志愿者被纳入研究,并在 2 周内接受了131 I-meplazumab的单光子发射计算机断层扫描 (SPECT) 成像。小鼠和人类中的分布与CD147的体内分布一致。生物分布和 SPECT 成像结果表明,肝脏是小鼠和人类吸收最高的器官。体内可观察到131 I-meplazumab的脱碘作用,服用 Lugol 溶液可有效保护甲状腺。131 I-meplazumab 在小鼠和人类中的药代动力学特征最符合两室模型。小鼠和人类的清除半衰期(T 1/2 β)分别为 117.4 和 223.5 小时。结果表明其体内药代动力学特性较为理想。健康志愿者计算出的有效剂量为0.811±0.260 mSv·MBq –1,是小鼠计算值的两倍。卢戈氏溶液封堵甲状腺后,使用诊断剂量的131 I-meplazumab进行人体临床影像实验是安全可行的。该研究将为推进meplazumab的临床转化提供更多的实验依据,对于评估COVID-19患者的治疗干预措施具有重要价值,也为其他抗体药物的临床转化研究提供参考。

































 京公网安备 11010802027423号
京公网安备 11010802027423号