Chemosphere ( IF 8.1 ) Pub Date : 2023-01-12 , DOI: 10.1016/j.chemosphere.2023.137866 Xiaoliang Guo , Qinqin Jiang , Zengru Li , Cai Cheng , Yu Feng , Yanlin He , Lingzi Zuo , Wei Ding , Delin Zhang , Lingling Feng
|
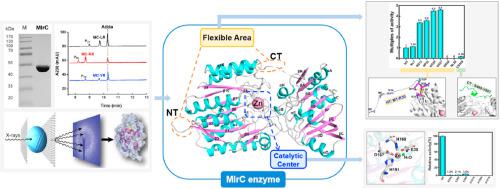
Microcystinase C (MlrC), one key hydrolase of the microcystinase family, plays an important role in linearized microsystin (L-MC) degradation. However, the three-dimensional structure and structural features of MlrC are still unclear. This study obtained high specific activity and high purity of MlrC by heterologous expression, and revealed that MlrC derived from Sphingomonas sp. ACM-3962 (ACM-MlrC) can degrade linearized products of MC-LR, MC-RR and MC-YR to product 3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid (Adda), indicating the degradation function and significance in MC-detoxification. More importantly, this study reported the crystal structure of ACM-MlrC at 2.6 Å resolution for the first time, which provides a basis for further understanding the structural characteristics and functions of MlrC. MlrC had a dual-domain feature, namely N and C terminal domain respectively. The N-terminal domain contained a Glutamate-Aspartate-Histidine-Histidine catalytic quadruplex coordinated with zinc ion in each monomer. The importance of zinc ions and their coordinated residues was analyzed by dialysis and site-directed mutagenesis methods. Moreover, the important influence of the N/C-terminal flexible regions of ACM-MlrC was also analyzed by sequence truncation, and then the higher yield and total activity of variants were obtained, which was beneficial to study the better function and application of MlrC.
中文翻译:

鞘氨醇单胞菌 MlrC 酶的晶体结构分析和表征。ACM-3962 参与线性化微囊藻毒素降解
微囊藻毒素 C (MlrC) 是微囊藻毒素家族的一种关键水解酶,在线性化微囊藻毒素 (L-MC) 降解中起着重要作用。然而,MlrC的三维结构和结构特征仍不清楚。本研究通过异源表达获得了高比活、高纯度的MlrC,揭示了MlrC来源于鞘氨醇单胞菌sp。ACM-3962 (ACM-MlrC) 可将 MC-LR、MC-RR 和 MC-YR 的线性化产物降解为产物 3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-二烯酸 (Adda),表明其在 MC 解毒中的降解功能和意义。更重要的是,本研究首次在2.6 Å分辨率下报道了ACM-MlrC的晶体结构,为进一步了解MlrC的结构特征和功能提供了基础。MlrC 具有双域特征,即分别为 N 和 C 末端域。N号-末端结构域包含与每个单体中的锌离子配位的谷氨酸-天冬氨酸-组氨酸-组氨酸催化四链体。通过透析和定点诱变方法分析了锌离子及其配位残基的重要性。此外,还通过序列截断分析了ACM-MlrC的N/C端柔性区的重要影响,进而获得了更高的产量和总活性的变异体,有利于更好地研究MlrC的功能和应用.

































 京公网安备 11010802027423号
京公网安备 11010802027423号