当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ferroxidase Activity in Eukaryotic Ferritin is Controlled by Accessory‐Iron‐Binding Sites in the Catalytic Cavity
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2016-09-21 , DOI: 10.1002/chem.201602842 Caterina Bernacchioni 1 , Cecilia Pozzi 2 , Flavio Di Pisa 2 , Stefano Mangani 2 , Paola Turano 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2016-09-21 , DOI: 10.1002/chem.201602842 Caterina Bernacchioni 1 , Cecilia Pozzi 2 , Flavio Di Pisa 2 , Stefano Mangani 2 , Paola Turano 1
Affiliation
|
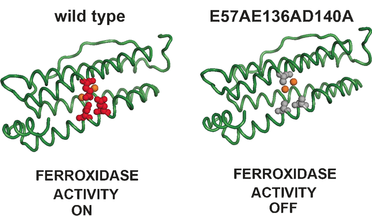
Ferritins are iron‐storage nanocage proteins that catalyze the oxidation of Fe2+ to Fe3+ at ferroxidase sites. By a combination of structural and spectroscopic techniques, Asp140, together with previously identified Glu57 and Glu136, is demonstrated to be an essential residue to promote the iron oxidation at the ferroxidase site. However, the presence of these three carboxylate moieties in close proximity to the catalytic centers is not essential to achieve binding of the Fe2+ substrate to the diferric ferroxidase sites with the same coordination geometries as in the wild‐type cages.
中文翻译:

真核铁蛋白中的铁氧化酶活性受催化腔中附件铁结合位点的控制
铁蛋白是铁存储的纳米笼蛋白,可在铁氧化酶位点催化Fe 2+氧化为Fe 3+。通过结构和光谱技术的结合,Asp140与先前鉴定的Glu57和Glu136一起被证明是促进铁氧化酶位点铁氧化的必需残基。但是,在靠近催化中心的位置存在这三个羧酸根部分对于以与野生型笼中相同的配位几何形状实现Fe 2+底物与二价铁氧化铁酶位点的结合并不是必不可少的。
更新日期:2016-09-21
中文翻译:

真核铁蛋白中的铁氧化酶活性受催化腔中附件铁结合位点的控制
铁蛋白是铁存储的纳米笼蛋白,可在铁氧化酶位点催化Fe 2+氧化为Fe 3+。通过结构和光谱技术的结合,Asp140与先前鉴定的Glu57和Glu136一起被证明是促进铁氧化酶位点铁氧化的必需残基。但是,在靠近催化中心的位置存在这三个羧酸根部分对于以与野生型笼中相同的配位几何形状实现Fe 2+底物与二价铁氧化铁酶位点的结合并不是必不可少的。















































 京公网安备 11010802027423号
京公网安备 11010802027423号