当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Accurate Potentials of Hg/HgO Electrodes: Practical Parameters for Reporting Alkaline Water Electrolysis Overpotentials
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-01-18 , DOI: 10.1021/acscatal.2c05655 Kenta Kawashima 1 , Raúl A. Márquez 1 , Yoon Jun Son 2 , Clarissa Guo 2 , Rinish Reddy Vaidyula 1 , Lettie A. Smith 1 , Chikaodili E. Chukwuneke 1 , C. Buddie Mullins 1, 2, 3, 4, 5
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-01-18 , DOI: 10.1021/acscatal.2c05655 Kenta Kawashima 1 , Raúl A. Márquez 1 , Yoon Jun Son 2 , Clarissa Guo 2 , Rinish Reddy Vaidyula 1 , Lettie A. Smith 1 , Chikaodili E. Chukwuneke 1 , C. Buddie Mullins 1, 2, 3, 4, 5
Affiliation
|
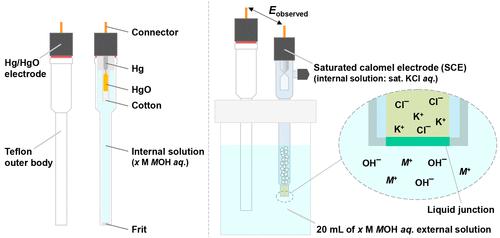
Figure 1. Left: External and internal views of the Hg/HgO reference electrode. Right: Two-electrode system for the Hg/HgO reference electrode potential determination. The potentials of the Hg/HgO electrodes were determined by using the calomel electrode with a 0.1 or 1 M KCl internal aqueous solution. (3) Liquid junction potentials were calculated through the Henderson equation. (35) Liquid junction potential was calculated according to the stationary Nernst–Planck equation (36) using LJPcalc software (https://swharden.com/LJPcalc). Figure 2. 95% confidence interval (CI) plots for the experimentally obtained potentials of Hg/HgO electrodes with different internal solutions (0.1–1 M NaOH/KOH). Dashed orange lines indicate the calculated Hg/HgO electrode potentials. Here, 95% probability experiment objective values lie in these as-calculated intervals. Note that experimental values are statistically compared with calculated values (≈ true values) in these figures. As seen in Table 3, if one uses two different alkaline solutions as internal and external electrolytes, undesired liquid junction potentials will be established at the Hg/HgO electrode frit. Moreover, during a long-term electrochemical test, internal/external solution may flow through the frit pores, resulting in the cation (Na+/K+) contamination and the concentration change of internal and external solutions. (38) Thus, one should use alkaline solutions with the same constituents at the same concentrations for both internal and external electrolytes. Since yellow HgO is highly soluble in aqueous solutions at low pH values (<4), (12,39) the use of Hg/HgO electrodes in acidic media should be avoided. A small amount of yellow HgO can also dissolve into alkaline solutions. (40) Thus, laboratory safety regulations with instructions for mercury-containing wastes should be followed when disposing of external electrolytes and especially internal solutions. As the Hg/HgO electrode can be electrochemically stable under temperatures up to 90 °C, (13,15−17) one can use the Hg/HgO electrode for electrochemical studies that require relatively hot environments (e.g., alkaline fuel cells, (41) methane electrooxidation systems, (42) etc.). Liquid junction potentials were calculated through the Henderson equation. (35) Liquid junction potentials were calculated according to the stationary Nernst–Planck equation (36) using LJPcalc software (https://swharden.com/LJPcalc). The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.2c05655. Experimental details, a scheme (experimental design), calculation details, digital photographs, tables (calculation parameters and results), time–potential curves (SCE vs Hg/HgO), SEM and EDX elemental mapping images, and EDX spectra (PDF) Most electronic Supporting Information files are available without a subscription to ACS Web Editions. Such files may be downloaded by article for research use (if there is a public use license linked to the relevant article, that license may permit other uses). Permission may be obtained from ACS for other uses through requests via the RightsLink permission system: http://pubs.acs.org/page/copyright/permissions.html. The authors gratefully acknowledge the support of the National Science Foundation (NSF) via Grant CHE-2102307. We also recognize the Welch Foundation for its generous support through Grant F-1436. This article references 42 other publications. This article has not yet been cited by other publications. Figure 1. Left: External and internal views of the Hg/HgO reference electrode. Right: Two-electrode system for the Hg/HgO reference electrode potential determination. Figure 2. 95% confidence interval (CI) plots for the experimentally obtained potentials of Hg/HgO electrodes with different internal solutions (0.1–1 M NaOH/KOH). Dashed orange lines indicate the calculated Hg/HgO electrode potentials. Here, 95% probability experiment objective values lie in these as-calculated intervals. Note that experimental values are statistically compared with calculated values (≈ true values) in these figures. This article references 42 other publications. The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.2c05655. Experimental details, a scheme (experimental design), calculation details, digital photographs, tables (calculation parameters and results), time–potential curves (SCE vs Hg/HgO), SEM and EDX elemental mapping images, and EDX spectra (PDF) Most electronic Supporting Information files are available without a subscription to ACS Web Editions. Such files may be downloaded by article for research use (if there is a public use license linked to the relevant article, that license may permit other uses). Permission may be obtained from ACS for other uses through requests via the RightsLink permission system:
http://pubs.acs.org/page/copyright/permissions.html.
中文翻译:

Hg/HgO 电极的准确电位:报告碱性水电解过电位的实用参数
图 1. 左:Hg/HgO 参比电极的外部和内部视图。右图:用于 Hg/HgO 参比电极电位测定的双电极系统。Hg/HgO 电极的电势通过使用带有 0.1 或 1 M KCl 内部水溶液的甘汞电极来确定。(3) 液接电位通过亨德森方程计算。(35) 使用 LJPcalc 软件 (https://swharden.com/LJPcalc) 根据稳态 Nernst–Planck 方程 (36) 计算液接电位。图 2. 实验获得的具有不同内部溶液 (0.1–1 M NaOH/KOH) 的 Hg/HgO 电极电位的 95% 置信区间 (CI) 图。橙色虚线表示计算出的 Hg/HgO 电极电位。这里,95% 的概率实验目标值位于这些计算的区间内。请注意,在这些图中,实验值与计算值(≈ 真实值)进行了统计比较。如表 3 所示,如果使用两种不同的碱性溶液作为内部和外部电解质,则将在 Hg/HgO 电极熔块处建立不需要的液接电位。此外,在长期的电化学测试中,内部/外部溶液可能流过玻璃料孔,导致阳离子(Na+ /K +) 污染和内部和外部溶液的浓度变化。(38) 因此,对于内部和外部电解质,应该使用具有相同成分、相同浓度的碱性溶液。由于黄色 HgO 在低 pH 值 (<4) 的水溶液中高度可溶,(12,39) 应避免在酸性介质中使用 Hg/HgO 电极。少量黄色HgO也能溶于碱性溶液。(40) 因此,在处理外部电解质,尤其是内部溶液时,应遵循实验室安全条例和含汞废物的说明。由于 Hg/HgO 电极在高达 90 °C 的温度下可以保持电化学稳定,(13,15-17) 人们可以使用 Hg/HgO 电极进行需要相对热环境的电化学研究(例如,碱性燃料电池,(41)甲烷电氧化系统,(42)等)。通过亨德森方程计算液接电位。(35) 使用 LJPcalc 软件 (https://swharden.com/LJPcalc) 根据稳态 Nernst–Planck 方程 (36) 计算液接电位。支持信息可在 https://pubs.acs.org/doi/10.1021/acscatal.2c05655 免费获得。实验细节、方案(实验设计)、计算细节、数码照片、表格(计算参数和结果)、时间-电位曲线(SCE vs Hg/HgO)、SEM 和 EDX 元素映射图像以及 EDX 光谱 (PDF) 大多数无需订阅 ACS 网络版即可获得电子支持信息文件。此类文件可以按文章下载以供研究使用(如果相关文章有链接的公共使用许可,则该许可可能允许其他用途)。可以通过 RightsLink 许可系统请求从 ACS 获得用于其他用途的许可:http://pubs.acs.org/page/copyright/permissions.html。作者非常感谢国家科学基金会 (NSF) 通过 Grant CHE-2102307 提供的支持。我们还感谢韦尔奇基金会通过 Grant F-1436 提供的慷慨支持。本文引用了 42 篇其他出版物。这篇文章尚未被其他出版物引用。图 1. 左:Hg/HgO 参比电极的外部和内部视图。右图:用于 Hg/HgO 参比电极电位测定的双电极系统。图 2。具有不同内部溶液(0.1–1 M NaOH/KOH)的 Hg/HgO 电极实验获得的电位的 95% 置信区间 (CI) 图。橙色虚线表示计算出的 Hg/HgO 电极电位。在这里,95% 的概率实验目标值位于这些计算间隔内。请注意,在这些图中,实验值与计算值(≈ 真实值)进行了统计比较。本文引用了 42 篇其他出版物。支持信息可在 https://pubs.acs.org/doi/10.1021/acscatal.2c05655 免费获得。实验细节、方案(实验设计)、计算细节、数码照片、表格(计算参数和结果)、时间-电位曲线(SCE vs Hg/HgO)、SEM 和 EDX 元素映射图像,和 EDX 光谱 (PDF) 大多数电子支持信息文件无需订阅 ACS 网络版即可获得。此类文件可以按文章下载以供研究使用(如果相关文章有链接的公共使用许可,则该许可可能允许其他用途)。可以通过 RightsLink 许可系统请求从 ACS 获得用于其他用途的许可:http://pubs.acs.org/page/copyright/permissions.html。
更新日期:2023-01-18
中文翻译:

Hg/HgO 电极的准确电位:报告碱性水电解过电位的实用参数
图 1. 左:Hg/HgO 参比电极的外部和内部视图。右图:用于 Hg/HgO 参比电极电位测定的双电极系统。Hg/HgO 电极的电势通过使用带有 0.1 或 1 M KCl 内部水溶液的甘汞电极来确定。(3) 液接电位通过亨德森方程计算。(35) 使用 LJPcalc 软件 (https://swharden.com/LJPcalc) 根据稳态 Nernst–Planck 方程 (36) 计算液接电位。图 2. 实验获得的具有不同内部溶液 (0.1–1 M NaOH/KOH) 的 Hg/HgO 电极电位的 95% 置信区间 (CI) 图。橙色虚线表示计算出的 Hg/HgO 电极电位。这里,95% 的概率实验目标值位于这些计算的区间内。请注意,在这些图中,实验值与计算值(≈ 真实值)进行了统计比较。如表 3 所示,如果使用两种不同的碱性溶液作为内部和外部电解质,则将在 Hg/HgO 电极熔块处建立不需要的液接电位。此外,在长期的电化学测试中,内部/外部溶液可能流过玻璃料孔,导致阳离子(Na+ /K +) 污染和内部和外部溶液的浓度变化。(38) 因此,对于内部和外部电解质,应该使用具有相同成分、相同浓度的碱性溶液。由于黄色 HgO 在低 pH 值 (<4) 的水溶液中高度可溶,(12,39) 应避免在酸性介质中使用 Hg/HgO 电极。少量黄色HgO也能溶于碱性溶液。(40) 因此,在处理外部电解质,尤其是内部溶液时,应遵循实验室安全条例和含汞废物的说明。由于 Hg/HgO 电极在高达 90 °C 的温度下可以保持电化学稳定,(13,15-17) 人们可以使用 Hg/HgO 电极进行需要相对热环境的电化学研究(例如,碱性燃料电池,(41)甲烷电氧化系统,(42)等)。通过亨德森方程计算液接电位。(35) 使用 LJPcalc 软件 (https://swharden.com/LJPcalc) 根据稳态 Nernst–Planck 方程 (36) 计算液接电位。支持信息可在 https://pubs.acs.org/doi/10.1021/acscatal.2c05655 免费获得。实验细节、方案(实验设计)、计算细节、数码照片、表格(计算参数和结果)、时间-电位曲线(SCE vs Hg/HgO)、SEM 和 EDX 元素映射图像以及 EDX 光谱 (PDF) 大多数无需订阅 ACS 网络版即可获得电子支持信息文件。此类文件可以按文章下载以供研究使用(如果相关文章有链接的公共使用许可,则该许可可能允许其他用途)。可以通过 RightsLink 许可系统请求从 ACS 获得用于其他用途的许可:http://pubs.acs.org/page/copyright/permissions.html。作者非常感谢国家科学基金会 (NSF) 通过 Grant CHE-2102307 提供的支持。我们还感谢韦尔奇基金会通过 Grant F-1436 提供的慷慨支持。本文引用了 42 篇其他出版物。这篇文章尚未被其他出版物引用。图 1. 左:Hg/HgO 参比电极的外部和内部视图。右图:用于 Hg/HgO 参比电极电位测定的双电极系统。图 2。具有不同内部溶液(0.1–1 M NaOH/KOH)的 Hg/HgO 电极实验获得的电位的 95% 置信区间 (CI) 图。橙色虚线表示计算出的 Hg/HgO 电极电位。在这里,95% 的概率实验目标值位于这些计算间隔内。请注意,在这些图中,实验值与计算值(≈ 真实值)进行了统计比较。本文引用了 42 篇其他出版物。支持信息可在 https://pubs.acs.org/doi/10.1021/acscatal.2c05655 免费获得。实验细节、方案(实验设计)、计算细节、数码照片、表格(计算参数和结果)、时间-电位曲线(SCE vs Hg/HgO)、SEM 和 EDX 元素映射图像,和 EDX 光谱 (PDF) 大多数电子支持信息文件无需订阅 ACS 网络版即可获得。此类文件可以按文章下载以供研究使用(如果相关文章有链接的公共使用许可,则该许可可能允许其他用途)。可以通过 RightsLink 许可系统请求从 ACS 获得用于其他用途的许可:http://pubs.acs.org/page/copyright/permissions.html。






























 京公网安备 11010802027423号
京公网安备 11010802027423号