当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Study for Plasma Assisted Cracking of NH3: Approaches and Challenges
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2023-01-19 , DOI: 10.1021/acs.jpca.2c06919 Seunghwan Bang 1 , Ramses Snoeckx 1 , Min Suk Cha 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2023-01-19 , DOI: 10.1021/acs.jpca.2c06919 Seunghwan Bang 1 , Ramses Snoeckx 1 , Min Suk Cha 1
Affiliation
|
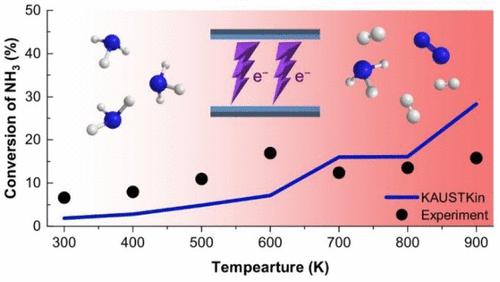
Ammonia is considered as one of the promising hydrogen carriers toward a sustainable world. Plasma assisted decomposition of NH3 could provide cost- and energy-effective, low-temperature, on-demand (partial) cracking of NH3 into H2. Here, we presented a temperature-dependent plasma-chemical kinetic study to investigate the role of both electron-induced reactions and thermally induced reactions on the decomposition of NH3. We employed a plasma-chemical kinetic model (KAUSTKin), developed a plasma-chemical reaction mechanism for the numerical analysis, and introduced a temperature-controlled dielectric barrier discharge reactor for the experimental investigation using 1 mol % NH3 diluted in N2. As a result, we observed the plasma significantly lowered the cracking temperature and found that the plasma-chemical mechanism should be further improved to better predict the experiment. The commonly used rates for the key NH3 pyrolysis reaction (NH3 + M ↔ NH2 + H + M) significantly overpredicted the recombination rate at temperatures below 600 K. Furthermore, the other identified shortcomings in the available data are (i) thermal hydrazine chemistry, (ii) electron-scattering cross-section data of NxHy, (iii) electron-impact dissociation of N2, and (iv) dissociative quenching of excited states of N2. We believe that the present study will spark fundamental interest to address these shortcomings and contribute to technical advancements in plasma assisted NH3 cracking technology.
中文翻译:

等离子体辅助裂解 NH3 的动力学研究:方法和挑战
氨被认为是实现可持续发展世界的有前途的氢载体之一。NH 3的等离子体辅助分解可以提供将NH 3成本和能量有效地、低温、按需(部分)裂解成H 2的方法。在这里,我们提出了一项温度依赖性等离子体化学动力学研究,以研究电子诱导反应和热诱导反应对 NH 3分解的作用。我们采用了等离子体化学动力学模型 (KAUSTKin),开发了用于数值分析的等离子体化学反应机制,并引入了用于实验研究的温控介质阻挡放电反应器,使用稀释在 N 2中的 1 mol % NH 3. 结果,我们观察到等离子体显着降低了裂化温度,并发现等离子体化学机制应进一步改进以更好地预测实验。关键 NH 3热解反应的常用速率(NH 3 + M ↔ NH 2 + H + M) 显着高估了温度低于 600 K 时的重组速率。此外,现有数据中发现的其他缺点是 (i) 热肼化学,(ii) N x H y的电子散射截面数据,(iii) N 2的电子碰撞解离,以及 (iv) N 2激发态的解离猝灭. 我们相信,本研究将激发解决这些缺点的根本兴趣,并有助于等离子体辅助 NH 3裂解技术的技术进步。
更新日期:2023-01-19
中文翻译:

等离子体辅助裂解 NH3 的动力学研究:方法和挑战
氨被认为是实现可持续发展世界的有前途的氢载体之一。NH 3的等离子体辅助分解可以提供将NH 3成本和能量有效地、低温、按需(部分)裂解成H 2的方法。在这里,我们提出了一项温度依赖性等离子体化学动力学研究,以研究电子诱导反应和热诱导反应对 NH 3分解的作用。我们采用了等离子体化学动力学模型 (KAUSTKin),开发了用于数值分析的等离子体化学反应机制,并引入了用于实验研究的温控介质阻挡放电反应器,使用稀释在 N 2中的 1 mol % NH 3. 结果,我们观察到等离子体显着降低了裂化温度,并发现等离子体化学机制应进一步改进以更好地预测实验。关键 NH 3热解反应的常用速率(NH 3 + M ↔ NH 2 + H + M) 显着高估了温度低于 600 K 时的重组速率。此外,现有数据中发现的其他缺点是 (i) 热肼化学,(ii) N x H y的电子散射截面数据,(iii) N 2的电子碰撞解离,以及 (iv) N 2激发态的解离猝灭. 我们相信,本研究将激发解决这些缺点的根本兴趣,并有助于等离子体辅助 NH 3裂解技术的技术进步。

































 京公网安备 11010802027423号
京公网安备 11010802027423号