当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron-Catalyzed Synthesis of Benzosultams from N-Phenyl-N-(prop-2-yn-1-yl)benzenesulfonamide and Benzenesulfonyl Hydrazine
Organic Letters ( IF 4.9 ) Pub Date : 2023-01-19 , DOI: 10.1021/acs.orglett.2c04134 Mei Zhang 1 , Xiao Yu 1 , Wenjing Zhu 1 , Yi Liu 1 , Zenghui Zhang 1 , Jinbo Zhao 1 , Chengcai Xia 1 , Ke Li 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-01-19 , DOI: 10.1021/acs.orglett.2c04134 Mei Zhang 1 , Xiao Yu 1 , Wenjing Zhu 1 , Yi Liu 1 , Zenghui Zhang 1 , Jinbo Zhao 1 , Chengcai Xia 1 , Ke Li 1
Affiliation
|
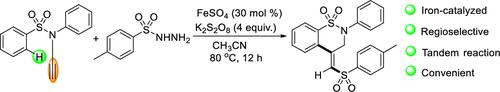
A novel route for an iron-catalyzed tandem sulfonylation, C(sp2)–H activation, cyclization reaction which uses N-phenyl-N-(prop-2-yn-1-yl)benzenesulfonamide and benzenesulfonohydrazide to synthesize derivatives of (Z)-2-phenyl-4-((phenylsulfonyl)methylene)-3,4-dihydro-2H-benzo[e][1,2]thiazine 1,1-dioxide has been developed. The method features convenient operation and good functional group tolerance. In addition, it employs insensitive and inexpensive FeSO4 as the catalyst and provides a direct approach for the preparation of benzothiazides.
中文翻译:

N-苯基-N-(prop-2-yn-1-yl)苯磺酰胺和苯磺酰肼铁催化合成苯磺胺
N-苯基-N- (prop-2-yn-1-yl)苯磺酰胺和苯磺酰肼铁催化串联磺酰化C(sp 2 )-H活化环化反应合成( Z )-2-phenyl-4-((phenylsulfonyl)methylene)-3,4-dihydro-2 H -benzo[ e ][1,2]thiazine 1,1-dioxide 已经开发出来。该方法操作方便,官能团耐受性好。此外,它采用不敏感且廉价的FeSO 4作为催化剂,为制备苯并噻嗪提供了一种直接的途径。
更新日期:2023-01-19
中文翻译:

N-苯基-N-(prop-2-yn-1-yl)苯磺酰胺和苯磺酰肼铁催化合成苯磺胺
N-苯基-N- (prop-2-yn-1-yl)苯磺酰胺和苯磺酰肼铁催化串联磺酰化C(sp 2 )-H活化环化反应合成( Z )-2-phenyl-4-((phenylsulfonyl)methylene)-3,4-dihydro-2 H -benzo[ e ][1,2]thiazine 1,1-dioxide 已经开发出来。该方法操作方便,官能团耐受性好。此外,它采用不敏感且廉价的FeSO 4作为催化剂,为制备苯并噻嗪提供了一种直接的途径。






























 京公网安备 11010802027423号
京公网安备 11010802027423号