Synthesis ( IF 2.2 ) Pub Date : 2023-01-18 , DOI: 10.1055/a-2002-5733
Yongjie Shi 1 , Nianxin Rong 2 , Xumu Zhang 1 , Qin Yin 2
|
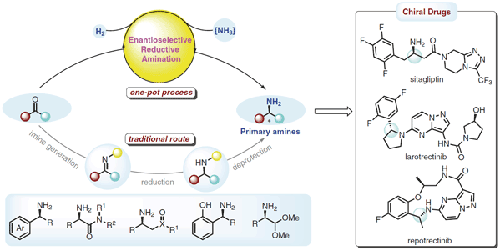
Chiral primary amines widely exist in drugs and are exceptionally important subunits or synthons in the syntheses of chiral secondary and tertiary amines of medicinal interest. Metal-catalyzed enantioselective reductive amination (ERA) of ketones with ammonium salts or ammonia provides a direct method for their synthesis. Although very useful, progress in this field has been very slow and important advances have only been achieved in the last few years. Several major challenges exist in this reaction, including (1) the reversible formation of unstable NH-imine intermediates; (2) the strong coordination property of N-containing reagents toward metal species; and (3) the lack of efficient catalytic systems that enable high enantiocontrol. Generally, the efficiency and enantiocontrol of this reaction is dependent on the substrate type, for instance, the use of α-keto esters/amides or aryl alkyl ketones is well established and they have even been used in the industrial production of chiral amine drugs. However, highly enantioselective control in dialkyl ketones, cyclic ketones, and α-keto acids remains unsolved. Herein, the historical development of ERA reactions with ammonium salts or ammonia gas is summarized, and novel synthetic applications toward useful synthons or drugs are presented. In addition, the factors restricting the growth of this method are also discussed.
1 Introduction
2 Enantioselective Reductive Amination via Hydrogenation
2.1 Enantioselective Reductive Amination of β-Keto Esters/Amides
2.2 Enantioselective Reductive Amination of Simple Ketones
2.3 Enantioselective Reductive Amination of α-Functionalized Ketones
2.4 Enantioselective Reductive Amination/Cyclization Cascade Reactions
2.5 Others
3 Enantioselective Reductive Amination via Transfer Hydrogenation
4 Synthetic Applications
5 Conclusions and Outlook
中文翻译:

通过对映选择性还原胺化合成手性伯胺:从学术界到工业界
手性伯胺广泛存在于药物中,是合成具有药用价值的手性仲胺和叔胺中极为重要的亚基或合成子。酮与铵盐或氨的金属催化对映选择性还原胺化 (ERA) 为其合成提供了直接方法。虽然非常有用,但该领域的进展非常缓慢,仅在最近几年才取得重要进展。该反应存在几个主要挑战,包括(1)不稳定NH-亚胺中间体的可逆形成;(2) 含氮试剂对金属物种的强配位性;(3) 缺乏能够实现高对映控制的高效催化系统。通常,该反应的效率和对映体控制取决于底物类型,例如,α-酮酯/酰胺或芳基烷基酮的使用已经很成熟,它们甚至已用于手性胺类药物的工业生产。然而,二烷基酮、环酮和 α-酮酸中的高对映选择性控制仍未解决。在此,总结了 ERA 反应与铵盐或氨气的历史发展,并介绍了对有用的合成子或药物的新合成应用。此外,还讨论了制约该方法发展的因素。总结了 ERA 与铵盐或氨气反应的历史发展,并介绍了对有用的合成子或药物的新合成应用。此外,还讨论了制约该方法发展的因素。总结了 ERA 与铵盐或氨气反应的历史发展,并介绍了对有用的合成子或药物的新合成应用。此外,还讨论了制约该方法发展的因素。
1 简介
2 氢化对映选择性还原胺化
2.1 β-酮酯/酰胺的对映选择性还原胺化
2.2 简单酮的对映选择性还原胺化
2.3 α-官能化酮的对映选择性还原胺化
2.4 对映选择性还原胺化/环化级联反应
2.5 其他
3 转移氢化对映选择性还原胺化
4 综合应用
5 结论与展望

































 京公网安备 11010802027423号
京公网安备 11010802027423号