当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iterative Design of Ionizable Lipids for Intramuscular mRNA Delivery
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-01-18 , DOI: 10.1021/jacs.2c10670 Grayson Tilstra 1 , Julien Couture-Senécal 1 , Yan Ming Anson Lau 1 , Alanna M Manning 1 , Daniel S M Wong 2 , Wanda W Janaeska 1 , Titobioluwa A Wuraola 1 , Janice Pang 1 , Omar F Khan 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-01-18 , DOI: 10.1021/jacs.2c10670 Grayson Tilstra 1 , Julien Couture-Senécal 1 , Yan Ming Anson Lau 1 , Alanna M Manning 1 , Daniel S M Wong 2 , Wanda W Janaeska 1 , Titobioluwa A Wuraola 1 , Janice Pang 1 , Omar F Khan 1, 3
Affiliation
|
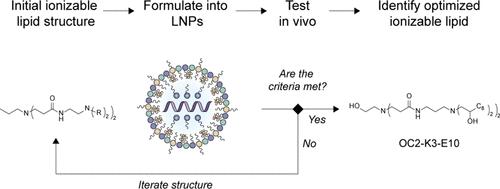
Lipid nanoparticles (LNPs) are the most clinically advanced delivery vehicles for RNA and have enabled the development of RNA-based drugs such as the mRNA COVID-19 vaccines. Functional delivery of mRNA by an LNP greatly depends on the inclusion of an ionizable lipid, and small changes to these lipid structures can significantly improve delivery. However, the structure–function relationships between ionizable lipids and mRNA delivery are poorly understood, especially for LNPs administered intramuscularly. Here, we show that the iterative design of a novel series of ionizable lipids generates key structure–activity relationships and enables the optimization of chemically distinct lipids with efficacy that is on-par with the current state of the art. We find that the combination of ionizable lipids comprising an ethanolamine core and LNPs with an apparent pKa between 6.6 and 6.9 maximizes intramuscular mRNA delivery. Furthermore, we report a nonlinear relationship between the lipid-to-mRNA mass ratio and protein expression, suggesting that a critical mass ratio exists for LNPs and may depend on ionizable lipid structure. Our findings add to the mechanistic understanding of ionizable lipids and demonstrate that hydrogen bonding, ionization behavior, and lipid-to-mRNA mass ratio are key design parameters affecting intramuscular mRNA delivery. We validate these insights by applying them to the rational design of new ionizable lipids. Overall, our iterative design strategy efficiently generates potent ionizable lipids. This hypothesis-driven method reveals structure–activity relationships that lay the foundation for the optimization of ionizable lipids in future LNP-RNA drugs. We foresee that this design strategy can be extended to other optimization parameters beyond intramuscular expression.
中文翻译:

用于肌内 mRNA 递送的可电离脂质的迭代设计
脂质纳米粒子 (LNPs) 是临床上最先进的 RNA 运载工具,并使基于 RNA 的药物(如 mRNA COVID-19 疫苗)的开发成为可能。LNP 对 mRNA 的功能性递送在很大程度上取决于可电离脂质的包含,对这些脂质结构的微小改变可以显着改善递送。然而,可电离脂质与 mRNA 递送之间的结构-功能关系知之甚少,尤其是对于肌肉注射的 LNP。在这里,我们展示了一系列新型可电离脂质的迭代设计产生了关键的结构-活性关系,并能够优化化学上不同的脂质,其功效与当前最先进的技术水平相当。A介于 6.6 和 6.9 之间可最大化肌内 mRNA 递送。此外,我们报告了脂质与 mRNA 质量比与蛋白质表达之间的非线性关系,表明 LNP 存在临界质量比,并且可能取决于可电离脂质结构。我们的研究结果增加了对可电离脂质的机械理解,并证明氢键、电离行为和脂质与 mRNA 的质量比是影响肌内 mRNA 递送的关键设计参数。我们通过将这些见解应用于新的可电离脂质的合理设计来验证这些见解。总的来说,我们的迭代设计策略有效地产生了有效的可电离脂质。这种假设驱动的方法揭示了构效关系,为未来 LNP-RNA 药物中可电离脂质的优化奠定了基础。
更新日期:2023-01-18
中文翻译:

用于肌内 mRNA 递送的可电离脂质的迭代设计
脂质纳米粒子 (LNPs) 是临床上最先进的 RNA 运载工具,并使基于 RNA 的药物(如 mRNA COVID-19 疫苗)的开发成为可能。LNP 对 mRNA 的功能性递送在很大程度上取决于可电离脂质的包含,对这些脂质结构的微小改变可以显着改善递送。然而,可电离脂质与 mRNA 递送之间的结构-功能关系知之甚少,尤其是对于肌肉注射的 LNP。在这里,我们展示了一系列新型可电离脂质的迭代设计产生了关键的结构-活性关系,并能够优化化学上不同的脂质,其功效与当前最先进的技术水平相当。A介于 6.6 和 6.9 之间可最大化肌内 mRNA 递送。此外,我们报告了脂质与 mRNA 质量比与蛋白质表达之间的非线性关系,表明 LNP 存在临界质量比,并且可能取决于可电离脂质结构。我们的研究结果增加了对可电离脂质的机械理解,并证明氢键、电离行为和脂质与 mRNA 的质量比是影响肌内 mRNA 递送的关键设计参数。我们通过将这些见解应用于新的可电离脂质的合理设计来验证这些见解。总的来说,我们的迭代设计策略有效地产生了有效的可电离脂质。这种假设驱动的方法揭示了构效关系,为未来 LNP-RNA 药物中可电离脂质的优化奠定了基础。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号