当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile and Scalable Synthesis of Self-Supported Zn-Doped CuO Nanosheet Arrays for Efficient Nitrate Reduction to Ammonium
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-01-17 , DOI: 10.1021/acsami.2c19011
Zhuzhu Du 1 , Kai Yang 1 , Hongfang Du 1, 2 , Boxin Li 1 , Ke Wang 1 , Song He 1 , Tingfeng Wang 1 , Wei Ai 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-01-17 , DOI: 10.1021/acsami.2c19011
Zhuzhu Du 1 , Kai Yang 1 , Hongfang Du 1, 2 , Boxin Li 1 , Ke Wang 1 , Song He 1 , Tingfeng Wang 1 , Wei Ai 1
Affiliation
|
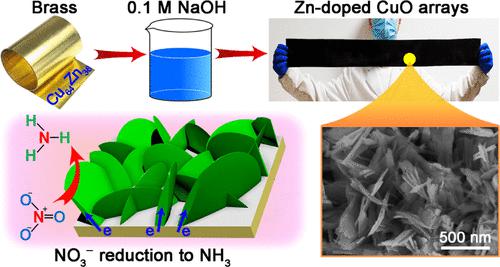
CuO has been regarded as a promising catalyst for the electrochemical reduction of nitrate (NO3–RR) to ammonium (NH3); however, the intrinsic activity is greatly restricted by its poor electrical property. In this work, self-supported Zn-doped CuO nanosheet arrays (Zn–CuO NAs) are synthesized for NO3–RR, where the Zn dopant regulates the electronic structure of CuO to significantly accelerate the interfacial charge transfer and inner electron transport kinetics. The Zn–CuO NAs are constructed by a one-step etching of commercial brass (Cu64Zn36 alloy) in 0.1 M NaOH solution, which experiences a corrosion–oxidation–reconstruction process. Initially, the brass undergoes a dealloying procedure to produce nanosized Cu, which is immediately oxidized to the Cu2O unit with a low valence state. Subsequently, Cu2O is further oxidized to the CuO unit and reconstructed into nanosheets with the coprecipitation of Zn2+. For NO3–RR, Zn–CuO NAs show a high NH3 production rate of 945.1 μg h–1 cm–2 and a Faradaic efficiency of up to 95.6% at −0.7 V in 0.1 M Na2SO4 electrolyte with 0.01 M NaNO3, which outperforms the majority of the state-of-the-art catalysts. The present work offers a facile yet very efficient strategy for the scale-up synthesis of Zn–CuO NAs for high-performance NH3 production from NO3–RR.
中文翻译:

自支撑 Zn 掺杂 CuO 纳米片阵列的简便和可扩展合成,用于将硝酸盐高效还原为铵
CuO 被认为是将硝酸盐 (NO 3 – RR) 电化学还原为铵 (NH 3 ) 的有前途的催化剂;然而,其固有活性受到其较差的电性能的极大限制。在这项工作中,合成了用于 NO 3 – RR的自支撑 Zn 掺杂 CuO 纳米片阵列 (Zn-CuO NAs) ,其中 Zn 掺杂剂调节 CuO 的电子结构以显着加速界面电荷转移和内部电子传输动力学。Zn-CuO NAs 通过一步蚀刻商业黄铜 (Cu 64 Zn 36合金)在 0.1 M NaOH 溶液中,经历了腐蚀-氧化-重建过程。最初,黄铜经过去合金化过程以产生纳米尺寸的 Cu,其立即被氧化成具有低价态的 Cu 2 O 单元。随后,Cu 2 O 被进一步氧化成 CuO 单元,并与 Zn 2+共沉淀重构为纳米片。对于 NO 3 – RR,Zn–CuO NAs在0.1 M Na 2 SO 4电解质和0.01 M NaNO 3,其性能优于大多数最先进的催化剂。目前的工作为从 NO 3 – RR中大规模合成 Zn-CuO NA 以实现高性能 NH 3生产提供了一种简单但非常有效的策略。
更新日期:2023-01-17
中文翻译:

自支撑 Zn 掺杂 CuO 纳米片阵列的简便和可扩展合成,用于将硝酸盐高效还原为铵
CuO 被认为是将硝酸盐 (NO 3 – RR) 电化学还原为铵 (NH 3 ) 的有前途的催化剂;然而,其固有活性受到其较差的电性能的极大限制。在这项工作中,合成了用于 NO 3 – RR的自支撑 Zn 掺杂 CuO 纳米片阵列 (Zn-CuO NAs) ,其中 Zn 掺杂剂调节 CuO 的电子结构以显着加速界面电荷转移和内部电子传输动力学。Zn-CuO NAs 通过一步蚀刻商业黄铜 (Cu 64 Zn 36合金)在 0.1 M NaOH 溶液中,经历了腐蚀-氧化-重建过程。最初,黄铜经过去合金化过程以产生纳米尺寸的 Cu,其立即被氧化成具有低价态的 Cu 2 O 单元。随后,Cu 2 O 被进一步氧化成 CuO 单元,并与 Zn 2+共沉淀重构为纳米片。对于 NO 3 – RR,Zn–CuO NAs在0.1 M Na 2 SO 4电解质和0.01 M NaNO 3,其性能优于大多数最先进的催化剂。目前的工作为从 NO 3 – RR中大规模合成 Zn-CuO NA 以实现高性能 NH 3生产提供了一种简单但非常有效的策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号