当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MnBr2-Catalyzed Aerobic Oxyazidation of Fluoroolefins: Access to Fluoroalkylated β-Hydroxy Aliphatic Azides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2023-01-17 , DOI: 10.1002/adsc.202201254
Yingying Cai 1 , Huanfeng Jiang 1 , Chuanle Zhu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2023-01-17 , DOI: 10.1002/adsc.202201254
Yingying Cai 1 , Huanfeng Jiang 1 , Chuanle Zhu 1
Affiliation
|
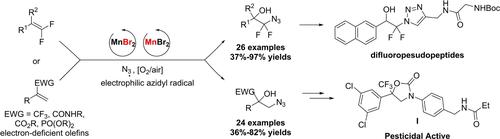
MnBr2-catalyzed oxyazidation of fluoroolefins with molecular oxygen and TMSN3 is reported. This method gives rise to various useful fluoroalkylated β-hydroxy aliphatic azides in 36%–97% yields. Importantly, this protocol features mild conditions, operationally simple, and gram-scalable, it tolerates various functional groups and has been applied in the synthesis of diverse attractive bioactive compounds and analogous. Mechanism studies enable the detection of the intermediate and indicate that a radical reaction process is involved. The β-fluoride elimination of α-fluoroalkylated radicals via radical-polar crossover pathway was completely inhibited in this reaction.
中文翻译:

MnBr2 催化的氟烯烃有氧叠氮化反应:获得氟烷基化 β-羟基脂肪族叠氮化物
报道了MnBr 2催化的氟代烯烃与分子氧和 TMSN 3的叠氮化反应。该方法以 36%–97% 的收率产生各种有用的氟烷基化 β-羟基脂肪族叠氮化物。重要的是,该协议具有温和的条件、操作简单和克级可扩展性,它可以容忍各种功能组,并已应用于各种有吸引力的生物活性化合物和类似物的合成。机理研究能够检测到中间体,并表明涉及一个自由基反应过程。α-氟烷基化自由基通过自由基-极性交叉途径的β-氟化物消除在该反应中被完全抑制。
更新日期:2023-01-17
中文翻译:

MnBr2 催化的氟烯烃有氧叠氮化反应:获得氟烷基化 β-羟基脂肪族叠氮化物
报道了MnBr 2催化的氟代烯烃与分子氧和 TMSN 3的叠氮化反应。该方法以 36%–97% 的收率产生各种有用的氟烷基化 β-羟基脂肪族叠氮化物。重要的是,该协议具有温和的条件、操作简单和克级可扩展性,它可以容忍各种功能组,并已应用于各种有吸引力的生物活性化合物和类似物的合成。机理研究能够检测到中间体,并表明涉及一个自由基反应过程。α-氟烷基化自由基通过自由基-极性交叉途径的β-氟化物消除在该反应中被完全抑制。







































 京公网安备 11010802027423号
京公网安备 11010802027423号