Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2023-01-18 , DOI: 10.1016/j.abb.2023.109517 Madison M Smith 1 , Dariush C Forouzesh 1 , Nicholas E Kaley 1 , Dali Liu 1 , Graham R Moran 1
|
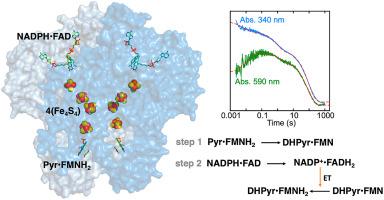
Dihydropyrimidine dehydrogenase (DPD) is a flavin dependent enzyme that catalyzes the reduction of the 5,6-vinylic bond of pyrimidines uracil and thymine with electrons from NADPH. DPD has two active sites that are separated by ∼60 Å. At one site NADPH binds adjacent to an FAD cofactor and at the other pyrimidine binds proximal to an FMN. Four Fe4S4 centers span the distance between these active sites. It has recently been established that the enzyme undergoes reductive activation prior to reducing the pyrimidine. In this initial process NADPH is oxidized at the FAD site and electrons are transmitted to the FMN via the Fe4S4 centers to yield the active state with a cofactor set of FAD•4(Fe4S4)•FMNH2. The catalytic chemistry of DPD can be studied in transient-state by observation of either NADPH consumption or charge transfer absorption associated with complexation of NADPH adjacent to the FAD. Here we have utilized both sets of absorption transitions to find evidence for specific additional aspects of the DPD mechanism. Competition for binding with NADP+ indicates that the two charge transfer species observed in activation/single turnover reactions arise from NADPH populating the FAD site before and after reductive activation. An additional charge transfer species is observed to accumulate at longer times when high NADPH concentrations are mixed with the enzyme•pyrimidine complex and this data can be modelled based on asymmetry in the homodimer. It was also shown that, like pyrimidines, dihydropyrimidines induce rapid reductive activation indicating that the reduced pyrimidine formed in turnover can stimulate the reinstatement of the active state of the enzyme. Investigation of the reverse reaction revealed that dihydropyrimidines alone can reductively activate the enzyme, albeit inefficiently. In the presence of dihydropyrimidine and NADP+ DPD will form NADPH but apparently without measurable reductive activation. Pyrimidines that have 5-substituent halogens were utilized to probe both reductive activation and turnover. The linearity of the Hammett plot based on the rate of hydride transfer to the pyrimidine establishes that, at least to the radius of an iodo-group, the 5-substituent volume does not have influence on the observed kinetics of pyrimidine reduction.
中文翻译:

哺乳动物二氢嘧啶脱氢酶:增加了电荷转移复合物瞬态分析的机制细节
二氢嘧啶脱氢酶 (DPD) 是一种黄素依赖性酶,它用来自 NADPH 的电子催化嘧啶尿嘧啶和胸腺嘧啶的 5,6-乙烯基键的还原。DPD 有两个活性位点,相隔 ~60 Å。在一个位点,NADPH 与 FAD 辅因子相邻结合,而在另一个位点,嘧啶与 FMN 近端结合。四个 Fe 4 S 4中心横跨这些活性位点之间的距离。最近已经确定该酶在还原嘧啶之前经历还原活化。在这个初始过程中,NADPH 在 FAD 位点被氧化,电子通过 Fe 4 S 4中心传输到 FMN,以产生具有 FAD•4(Fe 4 S 4辅助因子组的活性状态)•FMNH 2。DPD 的催化化学可以在瞬态下通过观察 NADPH 消耗或与 FAD 附近的 NADPH 络合相关的电荷转移吸收来研究。在这里,我们利用两组吸收转变来寻找 DPD 机制的特定附加方面的证据。与 NADP +结合的竞争表明在活化/单周转反应中观察到的两种电荷转移物种是由 NADPH 在还原活化前后填充 FAD 位点引起的。当高 NADPH 浓度与酶•嘧啶复合物混合时,观察到额外的电荷转移物质会在较长时间内积累,并且可以根据同二聚体中的不对称性对这一数据进行建模。还表明,与嘧啶一样,二氢嘧啶诱导快速还原活化,表明在周转过程中形成的还原嘧啶可以刺激酶活性状态的恢复。对逆反应的研究表明,单独的二氢嘧啶可以还原激活酶,尽管效率低下。在二氢嘧啶和 NADP +的存在下DPD 将形成 NADPH,但显然没有可测量的还原激活。具有 5 个取代基卤素的嘧啶被用于探测还原活化和周转。基于氢化物转移到嘧啶的速率的哈米特图的线性表明,至少在碘基的半径范围内,5-取代基体积对观察到的嘧啶还原动力学没有影响。































 京公网安备 11010802027423号
京公网安备 11010802027423号