Acta Biomaterialia ( IF 9.4 ) Pub Date : 2023-01-17 , DOI: 10.1016/j.actbio.2023.01.025 Seyed Mohammad Siadat 1 , Jeffrey W Ruberti 1
|
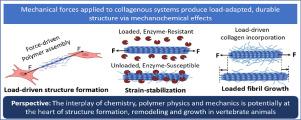
The collagen molecular family is the result of nearly one billion years of evolution. It is a unique family of proteins, the majority of which provide general mechanical support to biological tissues. Fibril forming collagens are the most abundant collagens in vertebrate animals and are generally found in positions that resist tensile loading. In animals, cells produce fibril-forming collagen molecules that self-assemble into larger structures known as collagen fibrils. Collagen fibrils are the fundamental, continuous, load-bearing elements in connective tissues, but are often further aggregated into larger load-bearing structures, fascicles in tendon, lamellae in cornea and in intervertebral disk. We know that failure to form fibrillar collagen is embryonic lethal, and excessive collagen formation/growth (fibrosis) or uncontrolled enzymatic remodeling (type II collagen: osteoarthritis) is pathological. Collagen is thus critical to vertebrate viability and instrumental in maintaining efficient mechanical structures. However, despite decades of research, our understanding of collagen matrix formation is not complete, and we know still less about the detailed mechanisms that drive collagen remodeling, growth, and pathology. In this perspective, we examine the known role of mechanical force on the formation and development of collagenous structure. We then discuss a mechanochemical mechanism that has the potential to unify our understanding of collagenous tissue assembly dynamics, which preferentially deposits and grows collagen fibrils directly in the path of mechanical force, where the energetics should be dissuasive and where collagen fibrils are most required. We term this mechanism: Mechanochemical force-structure causality.
Statement of significance
Our mechanochemical-force structure causality postulate suggests that collagen molecules are components of mechanochemically-sensitive and dynamically-responsive fibrils. Collagen molecules assemble preferentially in the path of applied strain, can be grown in place by mechanical extension, and are retained in the path of force through strain-stabilization. The mechanisms that drive this behavior operate at the level of the molecules themselves and are encoded into the structure of the biomaterial. The concept might change our understanding of structure formation, enhance our ability to treat injuries, and accelerate the development of therapeutics to prevent pathologies such as fibrosis. We suggest that collagen is a mechanochemically responsive dynamic element designed to provide a substantial “material assist” in the construction of adaptive carriers of mechanical signals.
中文翻译:

胶原蛋白的机械化学
胶原蛋白分子家族是近十亿年进化的结果。它是一个独特的蛋白质家族,其中大部分为生物组织提供一般机械支持。形成原纤维的胶原蛋白是脊椎动物中最丰富的胶原蛋白,通常存在于抵抗拉伸负荷的部位。在动物体内,细胞会产生形成原纤维的胶原蛋白分子,这些分子会自组装成更大的结构,称为胶原原纤维。胶原原纤维是结缔组织中基本的、连续的、承重的元素,但通常会进一步聚集成更大的承重结构,如肌腱中的束、角膜中的薄片和椎间盘中的。我们知道无法形成纤维状胶原蛋白对胚胎来说是致命的,过度的胶原蛋白形成/生长(纤维化)或不受控制的酶重塑(II 型胶原蛋白:骨关节炎)是病态的。因此,胶原蛋白对脊椎动物的生存能力至关重要,并且有助于维持有效的机械结构。然而,尽管进行了几十年的研究,我们对胶原蛋白基质形成的理解并不完整,而且我们对驱动胶原蛋白重塑、生长和病理的详细机制知之甚少。从这个角度来看,我们研究了机械力对胶原结构形成和发展的已知作用。然后我们讨论了一种机械化学机制,它有可能统一我们对胶原组织组装动力学的理解,它优先在机械力的路径上直接沉积和生长胶原纤维,能量学应该劝阻的地方和最需要胶原纤维的地方。我们将这种机制称为:机械化学力-结构因果关系。
重要性声明
我们的机械化学力结构因果关系假设表明,胶原分子是机械化学敏感和动态响应原纤维的组成部分。胶原蛋白分子优先在施加应变的路径中组装,可以通过机械延伸在适当的位置生长,并通过应变稳定保留在力的路径中。驱动这种行为的机制在分子本身的水平上运作,并被编码到生物材料的结构中。这个概念可能会改变我们对结构形成的理解,增强我们治疗损伤的能力,并加速治疗方法的发展以预防纤维化等病症。































 京公网安备 11010802027423号
京公网安备 11010802027423号