当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tailoring the Electronic Ru-Al2O3 Interaction to Regulate Reaction Barriers for Selective Hydrogenolysis of Aromatic Ether
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-01-17 , DOI: 10.1021/acssuschemeng.2c05987
Hongfei Xiao 1, 2 , Jianghao Zhang 2 , Chuo Du 2, 3 , Yanxia Zheng 2 , Jinhou Fang 4 , Shuang Li 1 , Changbin Zhang 2, 3, 4
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-01-17 , DOI: 10.1021/acssuschemeng.2c05987
Hongfei Xiao 1, 2 , Jianghao Zhang 2 , Chuo Du 2, 3 , Yanxia Zheng 2 , Jinhou Fang 4 , Shuang Li 1 , Changbin Zhang 2, 3, 4
Affiliation
|
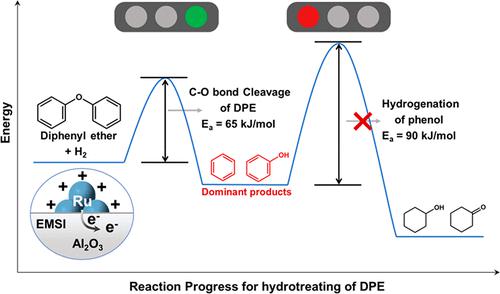
Aromatics are desirable products from the depolymerization/valorization of lignin; however, it is still challenging to achieve selective hydrogenolysis of the C–O bond with the preservation of aromatic rings. In this work, the electronic Ru-Al2O3 interaction was tailored by controlling the sizes of supported Ru nanoparticles to regulate the profiles of energy barriers for selective hydrogenolysis of diphenyl ether (DPE, modeling compound of lignin). Complementary characterizations and kinetic studies demonstrate that a stronger electronic metal–support interaction (EMSI) occurs between smaller Ru nanoparticles and Al2O3, leading to a more electron-deficient Ru domain. This tailored electronic structure dramatically increases the barrier of an undesired secondary reaction (i.e., ring hydrogenation), outstripping that of DPE hydrogenolysis for the production of aromatics. In addition, although the latter barrier also increases over smaller and more electron-deficient Ru, the much more abundant active site compensates for the increased barrier and enhanced the apparent reactivity. The catalyst bearing the smallest Ru particle displays the highest activity and selectivity under the tested conditions. This work provides an approach to control selectivity in hydrotreatment by regulating the energy barriers along the reaction pathway.
中文翻译:

调整电子 Ru-Al2O3 相互作用以调节芳香醚选择性氢解的反应势垒
芳烃是木质素解聚/增值的理想产物;然而,在保留芳环的情况下实现C-O键的选择性氢解仍然具有挑战性。在这项工作中,电子 Ru-Al 2 O 3相互作用是通过控制负载的 Ru 纳米粒子的大小来调节二苯醚(DPE,木质素的模型化合物)选择性氢解的能垒分布来定制的。互补表征和动力学研究表明,较小的 Ru 纳米颗粒和 Al 2 O 3之间会发生更强的电子金属-载体相互作用 (EMSI),导致更缺电子的 Ru 域。这种定制的电子结构显着增加了不需要的二次反应(即环氢化)的势垒,超过了 DPE 氢解生产芳烃的势垒。此外,尽管后一个势垒也随着更小和更缺电子的 Ru 而增加,但更丰富的活性位点补偿了势垒的增加并增强了表观反应性。带有最小 Ru 颗粒的催化剂在测试条件下显示出最高的活性和选择性。这项工作提供了一种通过调节反应路径上的能垒来控制加氢处理选择性的方法。
更新日期:2023-01-17
中文翻译:

调整电子 Ru-Al2O3 相互作用以调节芳香醚选择性氢解的反应势垒
芳烃是木质素解聚/增值的理想产物;然而,在保留芳环的情况下实现C-O键的选择性氢解仍然具有挑战性。在这项工作中,电子 Ru-Al 2 O 3相互作用是通过控制负载的 Ru 纳米粒子的大小来调节二苯醚(DPE,木质素的模型化合物)选择性氢解的能垒分布来定制的。互补表征和动力学研究表明,较小的 Ru 纳米颗粒和 Al 2 O 3之间会发生更强的电子金属-载体相互作用 (EMSI),导致更缺电子的 Ru 域。这种定制的电子结构显着增加了不需要的二次反应(即环氢化)的势垒,超过了 DPE 氢解生产芳烃的势垒。此外,尽管后一个势垒也随着更小和更缺电子的 Ru 而增加,但更丰富的活性位点补偿了势垒的增加并增强了表观反应性。带有最小 Ru 颗粒的催化剂在测试条件下显示出最高的活性和选择性。这项工作提供了一种通过调节反应路径上的能垒来控制加氢处理选择性的方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号