当前位置:
X-MOL 学术
›
ACS ES&T Water
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of Dichlorine Monoxide for Organic Pollutant Degradation by Free Chlorine in High Chloride-Containing Water
ACS ES&T Water ( IF 4.8 ) Pub Date : 2023-01-15 , DOI: 10.1021/acsestwater.2c00477 Chuanjing Lin 1 , Li Ling 1, 2 , Chii Shang 1, 3
ACS ES&T Water ( IF 4.8 ) Pub Date : 2023-01-15 , DOI: 10.1021/acsestwater.2c00477 Chuanjing Lin 1 , Li Ling 1, 2 , Chii Shang 1, 3
Affiliation
|
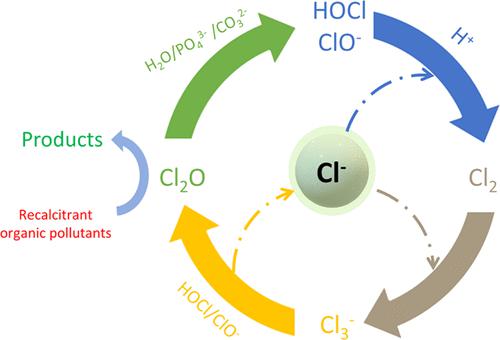
Several ppm levels of free chlorine in high chloride-containing water of no less than 0.20 M show remarkably high degradation rate constants toward carbamazepine (kCBZ′). Dichlorine monoxide (Cl2O) was identified to be responsible for the high kCBZ′. Cl2O degrades aromatic pollutants connected to more electron-donating functional groups faster when comparing kCl2O to 1,4-dimethoxybenzene, bisphenol A, benzoic acid, and CBZ. However, its formation at low free chlorine levels in high chloride-containing water cannot be explained by our current knowledge. We proposed an alternative Cl2O formation mechanism, in which chloride transforms hypochlorous acid/hypochlorite (HOCl/ClO–) to dichlorine (Cl2) and polychloride monoanions (Cln–), which then react with HOCl/ClO– to form Cl2O. Decreasing pH from 8.60 to 4.33 increases Cl2 concentrations and thus generates about 600× higher Cl2O concentrations. Weak acid anions, for example, phosphate and bicarbonate, are strong nucleophiles assisting the Cl2O hydrolysis, but their presence at the testing conditions does not much affect kCBZ′. The proposed alternative Cl2O formation mechanism reveals the roles of chloride in shifting the equilibrium toward the Cl2O formation, allowing the simple chlorine addition strategy to degrade recalcitrant organic pollutants in high chloride-containing wastewater. It may also change our understanding of the speciation of different free chlorine species and their impacts on the DBP formation in high chloride-containing wastewater.
中文翻译:

高氯水中游离氯降解有机污染物生成一氧化二氯
在不低于 0.20 M 的高氯化物水中,几个 ppm 水平的游离氯显示出对卡马西平的非常高的降解速率常数 ( k CBZ ')。一氧化二氯 (Cl 2 O) 被确定是造成高k CBZ ' 的原因。与 1,4-二甲氧基苯、双酚 A 、苯甲酸和 CBZ相比, Cl 2 O可以更快地降解与更多供电子官能团相连的芳香族污染物。然而,我们目前的知识无法解释它在高含氯水中的低游离氯水平下的形成。我们提出了另一种 Cl 2O 形成机制,其中氯化物将次氯酸/次氯酸盐 (HOCl/ClO – ) 转化为二氯 (Cl 2 ) 和多氯单阴离子 (Cl n – ),然后与 HOCl/ClO –反应形成 Cl 2 O。降低 pH 值8.60 至 4.33 增加了 Cl 2浓度,因此产生了约 600 倍高的 Cl 2 O 浓度。弱酸性阴离子,例如磷酸盐和碳酸氢盐,是有助于Cl 2 O水解的强亲核试剂,但它们在测试条件下的存在对k CBZ '影响不大。提议的替代品 Cl 2O 形成机制揭示了氯化物在使平衡向 Cl 2 O 形成方向移动中的作用,允许简单的氯添加策略来降解高氯废水中的顽固有机污染物。它还可能改变我们对不同游离氯物种的形态及其对高氯废水中 DBP 形成的影响的理解。
更新日期:2023-01-15
中文翻译:

高氯水中游离氯降解有机污染物生成一氧化二氯
在不低于 0.20 M 的高氯化物水中,几个 ppm 水平的游离氯显示出对卡马西平的非常高的降解速率常数 ( k CBZ ')。一氧化二氯 (Cl 2 O) 被确定是造成高k CBZ ' 的原因。与 1,4-二甲氧基苯、双酚 A 、苯甲酸和 CBZ相比, Cl 2 O可以更快地降解与更多供电子官能团相连的芳香族污染物。然而,我们目前的知识无法解释它在高含氯水中的低游离氯水平下的形成。我们提出了另一种 Cl 2O 形成机制,其中氯化物将次氯酸/次氯酸盐 (HOCl/ClO – ) 转化为二氯 (Cl 2 ) 和多氯单阴离子 (Cl n – ),然后与 HOCl/ClO –反应形成 Cl 2 O。降低 pH 值8.60 至 4.33 增加了 Cl 2浓度,因此产生了约 600 倍高的 Cl 2 O 浓度。弱酸性阴离子,例如磷酸盐和碳酸氢盐,是有助于Cl 2 O水解的强亲核试剂,但它们在测试条件下的存在对k CBZ '影响不大。提议的替代品 Cl 2O 形成机制揭示了氯化物在使平衡向 Cl 2 O 形成方向移动中的作用,允许简单的氯添加策略来降解高氯废水中的顽固有机污染物。它还可能改变我们对不同游离氯物种的形态及其对高氯废水中 DBP 形成的影响的理解。































 京公网安备 11010802027423号
京公网安备 11010802027423号