当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Streamlined Chemoenzymatic Synthesis of Cyclic Peptides by Non-ribosomal Peptide Cyclases
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-01-13 , DOI: 10.1021/jacs.2c11082 Masakazu Kobayashi 1 , Kei Fujita 1 , Kenichi Matsuda 1, 2 , Toshiyuki Wakimoto 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-01-13 , DOI: 10.1021/jacs.2c11082 Masakazu Kobayashi 1 , Kei Fujita 1 , Kenichi Matsuda 1, 2 , Toshiyuki Wakimoto 1, 2
Affiliation
|
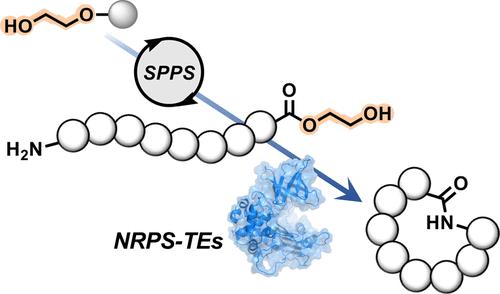
Macrocyclization improves the pharmaceutical properties of peptides; however, regio- and chemoselective intramolecular cyclizations remain challenging. Here we developed a streamlined chemoenzymatic approach to synthesize cyclic peptides by exploiting non-ribosomal peptide (NRP) cyclases. Linear peptides linked to the resin through a C-terminal diol ester functionality are synthesized on a solid support, to circumvent the installation of leaving groups to the peptidic substrates in the liquid phase which often triggers undesirable epimerization. Cleavage of the resin-bound peptides yielded the diol esters with sufficient purity to be readily cyclized in a head-to-tail manner by SurE, a representative penicillin-binding protein-type thioesterase (PBP-type TE). Explorations of homologous wild-type enzymes as well as rational protein engineering have broadened the scope of the enzymatic macrolactamization. This method will potentially accelerate the exploitation of NRP cyclases as biocatalysts.
中文翻译:

非核糖体肽环化酶对环肽的简化化学酶合成
大环化改善多肽的药学性质;然而,区域和化学选择性分子内环化仍然具有挑战性。在这里,我们开发了一种简化的化学酶法,通过利用非核糖体肽 (NRP) 环化酶来合成环肽。通过C连接到树脂的线性肽- 末端二醇酯功能在固体支持物上合成,以规避离去基团在液相中安装到肽底物上,这通常会引发不希望的差向异构化。树脂结合肽的裂解产生了具有足够纯度的二醇酯,可以很容易地被 SurE(一种代表性的青霉素结合蛋白型硫酯酶(PBP 型 TE))以头对尾的方式环化。对同源野生型酶的探索以及合理的蛋白质工程已经拓宽了酶促大环内酰胺化的范围。这种方法将有可能加速 NRP 环化酶作为生物催化剂的开发。
更新日期:2023-01-13
中文翻译:

非核糖体肽环化酶对环肽的简化化学酶合成
大环化改善多肽的药学性质;然而,区域和化学选择性分子内环化仍然具有挑战性。在这里,我们开发了一种简化的化学酶法,通过利用非核糖体肽 (NRP) 环化酶来合成环肽。通过C连接到树脂的线性肽- 末端二醇酯功能在固体支持物上合成,以规避离去基团在液相中安装到肽底物上,这通常会引发不希望的差向异构化。树脂结合肽的裂解产生了具有足够纯度的二醇酯,可以很容易地被 SurE(一种代表性的青霉素结合蛋白型硫酯酶(PBP 型 TE))以头对尾的方式环化。对同源野生型酶的探索以及合理的蛋白质工程已经拓宽了酶促大环内酰胺化的范围。这种方法将有可能加速 NRP 环化酶作为生物催化剂的开发。































 京公网安备 11010802027423号
京公网安备 11010802027423号