当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pd(0)-Catalyzed Asymmetric 7-Endo Hydroacyloxylative Cyclization of 1,6-Enyne Enabled by an Anion Ligand-Directed Strategy
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-01-13 , DOI: 10.1021/jacs.2c12756 Ming Dong 1, 2 , Linjun Qi 1 , Jinlong Qian 1 , Shuling Yu 1 , Xiaofeng Tong 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-01-13 , DOI: 10.1021/jacs.2c12756 Ming Dong 1, 2 , Linjun Qi 1 , Jinlong Qian 1 , Shuling Yu 1 , Xiaofeng Tong 1, 2
Affiliation
|
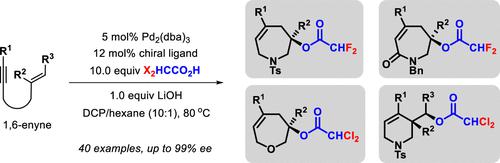
Despite diversity in reaction mechanisms, the palladium-catalyzed cyclization of 1,6-enyne generally proceeds in a 5-exo manner. Herein, we report the development of a Pd(0)-catalyzed hydroacyloxylative cyclization of 1,6-enyne in either 7-endo-trig or 6-exo-trig fashion when paired with an appropriate dihaloacetic acid reactant, such as F2HCCO2H and Cl2HCCO2H. Using the combination of Pd2(dba)3 and a chiral phosphine ligand, the hydroacyloxylative cyclization of 1,6-enyne bearing a 1,1-disubstituted alkene moiety readily gives highly enantiopure seven-membered heterocycles while the reaction of those having a 1,2-disubstituted alkene affords six-membered rings with moderate enantioselectivity. Preliminary experimental studies suggest a reaction mechanism featuring an unusual E-to-Z vinyl-Pd(II) isomerization and alkene trans-oxypalladation, which is proven to be governed by the rationally selected carboxylate.
中文翻译:

Pd(0)-催化的 1,6-烯炔的不对称 7-Endo Hydroacyloxylative 环化由阴离子配体导向策略实现
尽管反应机制多种多样,但钯催化的 1,6-烯炔环化通常以 5-外切方式进行。在此,我们报告了在与适当的二卤乙酸反应物(例如 F 2 HCCO )配对时,Pd(0) 催化的 1,6-烯炔以 7-内-触发或 6-外-触发方式的加氢酰氧基化环化的发展2 H和Cl 2 HCCO 2 H.结合使用Pd 2 (dba) 3和手性膦配体,带有 1,1-二取代烯烃部分的 1,6-烯炔的加氢酰氧基化环化很容易得到高度对映体纯的七元杂环,而具有 1,2-二取代烯烃的那些反应提供六元环具有适度的对映选择性。初步实验研究表明,反应机制具有不寻常的E -to- Z乙烯基-Pd (II) 异构化和烯烃反式氧钯化反应,这被证明是由合理选择的羧酸盐控制的。
更新日期:2023-01-13
中文翻译:

Pd(0)-催化的 1,6-烯炔的不对称 7-Endo Hydroacyloxylative 环化由阴离子配体导向策略实现
尽管反应机制多种多样,但钯催化的 1,6-烯炔环化通常以 5-外切方式进行。在此,我们报告了在与适当的二卤乙酸反应物(例如 F 2 HCCO )配对时,Pd(0) 催化的 1,6-烯炔以 7-内-触发或 6-外-触发方式的加氢酰氧基化环化的发展2 H和Cl 2 HCCO 2 H.结合使用Pd 2 (dba) 3和手性膦配体,带有 1,1-二取代烯烃部分的 1,6-烯炔的加氢酰氧基化环化很容易得到高度对映体纯的七元杂环,而具有 1,2-二取代烯烃的那些反应提供六元环具有适度的对映选择性。初步实验研究表明,反应机制具有不寻常的E -to- Z乙烯基-Pd (II) 异构化和烯烃反式氧钯化反应,这被证明是由合理选择的羧酸盐控制的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号