Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2023-01-14 , DOI: 10.1016/j.jhazmat.2023.130790
Xin Zhang 1 , Qingling Fu 1 , Hongqing Hu 1 , Jun Zhu 1 , Yonghong Liu 2
|
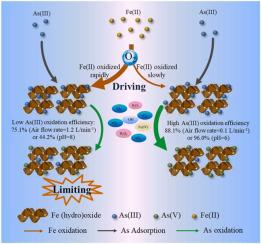
The co-oxidation of Fe(II) and As(III) occurs under aerobic conditions, and Fe(II) may largely determine the fate of As(III), but the effect of Fe(II) on the As(III) oxidation is barely explored. In this research, the limiting and driving roles of Fe(II) in As(III) oxidation were systematically studied through batch kinetic studies in combination with X-ray photoelectron spectroscopy (XPS) depth profiling, scanning electron microscopy and energy dispersive X-ray spectrometry (SEM-EDS), and quenching experiments. The results showed that As(III) oxidation efficiency increased with the increase of Fe/As molar ratio (from 63.1% to 98.3%), but decreased with the increase of pH (from 96.0% to 44.2%) and the increase of air flow rate (from 88.1% to 75.1%). The Fe(II) oxidation rate increased with the increase of pH and air flow rate. When Fe(II) was oxidized rapidly, As(III) was more likely to be immobilized in the “inner sphere” of formed Fe (hydr)oxides, limiting As(III) oxidation. On the other hand, Fe(II) was oxidized to produce Fe (hydr)oxides to adsorb or fix As(III); meanwhile, the ROS generated by Fenton-like reaction of Fe(II) promoted As(III) oxidation, especially, •O2− and H2O2 were important ROS that drove the As(III) oxidation. These findings might provide a new insight for Fe(II) and As(III) geochemistry cycling in naturally occurring environment.
中文翻译:

Fe(II) 对 Fe(II)-As(III) 共氧化中 As(III) 氧化的影响:限制和驱动作用
Fe(II) 和 As(III) 的共氧化发生在有氧条件下,Fe(II) 可能在很大程度上决定了 As(III) 的命运,但 Fe(II) 对 As(III) 氧化的影响几乎没有被探索过。在这项研究中,通过批量动力学研究结合 X 射线光电子能谱 (XPS) 深度剖析、扫描电子显微镜和能量色散 X 射线,系统地研究了 Fe(II) 在 As(III) 氧化中的限制和驱动作用光谱法 (SEM-EDS) 和淬火实验。结果表明,As(III) 氧化效率随着 Fe/As 摩尔比的增加(从 63.1% 到 98.3%)而增加,但随着 pH 值的增加(从 96.0% 到 44.2%)和空气流量的增加而降低率(从 88.1% 到 75.1%)。Fe(II) 氧化速率随着 pH 值和空气流速的增加而增加。当 Fe(II) 被快速氧化时,As(III) 更有可能固定在形成的 Fe(氢氧化物)的“内球”中,从而限制 As(III) 的氧化。另一方面,Fe(II)被氧化生成Fe(hydro)oxide来吸附或固定As(III);同时,Fe(II)类芬顿反应产生的ROS促进了As(III)的氧化,尤其是•O2 -和 H 2 O 2是驱动 As(III) 氧化的重要 ROS。这些发现可能为自然环境中的 Fe(II) 和 As(III) 地球化学循环提供新的见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号