当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-light-induced direct perfluoroalkylation/heteroarylation of [1.1.1]propellane to diverse bicyclo[1.1.1]pentanes (BCPs) under metal and photocatalyst-free conditions
Green Chemistry ( IF 9.3 ) Pub Date : 2023-01-12 , DOI: 10.1039/d2gc04521d Jiashun Zhu 1 , Yirui Guo 1 , Yuru Zhang 1 , Wanmei Li 1 , Pengfei Zhang 1 , Jun Xu 1
Green Chemistry ( IF 9.3 ) Pub Date : 2023-01-12 , DOI: 10.1039/d2gc04521d Jiashun Zhu 1 , Yirui Guo 1 , Yuru Zhang 1 , Wanmei Li 1 , Pengfei Zhang 1 , Jun Xu 1
Affiliation
|
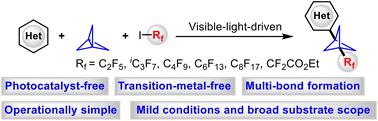
Herein, a green and novel multi-component reaction for direct perfluoroalkylation/heteroarylation of [1.1.1]propellane with heteroarenes and perfluoroalkyl iodines has been described. This transformation is facilitated by visible light in the absence of any transition-metal catalysts and photocatalysts. Various heteroarenes and perfluoroalkyl iodines are well compatible, providing the corresponding products in moderate-to-good yields. The control experiments demonstrate that a radical-relay mechanism is responsible for the cascade reaction. Such a methodology offers an efficient and practical access to diverse 1-perfluoroalkyl-3-heteroaryl bicyclo[1.1.1]pentanes (BCPs) with the potential for various applications in organic synthesis.
中文翻译:

在无金属和无光催化剂的条件下,可见光诱导的 [1.1.1] 丙烷直接全氟烷基化/杂芳基化为多种双环 [1.1.1] 戊烷 (BCP)
在此,描述了 [1.1.1] 丙烷与杂芳烃和全氟烷基碘的直接全氟烷基化/杂芳基化的绿色新型多组分反应。在没有任何过渡金属催化剂和光催化剂的情况下,可见光促进了这种转化。各种杂芳烃和全氟烷基碘具有很好的相容性,以中等到良好的收率提供相应的产品。控制实验表明自由基中继机制负责级联反应。这种方法提供了一种有效且实用的方法来获得各种 1-perfluoroalkyl-3-heteroaryl 双环 [1.1.1] 戊烷 (BCP),并具有在有机合成中的各种应用潜力。
更新日期:2023-01-12
中文翻译:

在无金属和无光催化剂的条件下,可见光诱导的 [1.1.1] 丙烷直接全氟烷基化/杂芳基化为多种双环 [1.1.1] 戊烷 (BCP)
在此,描述了 [1.1.1] 丙烷与杂芳烃和全氟烷基碘的直接全氟烷基化/杂芳基化的绿色新型多组分反应。在没有任何过渡金属催化剂和光催化剂的情况下,可见光促进了这种转化。各种杂芳烃和全氟烷基碘具有很好的相容性,以中等到良好的收率提供相应的产品。控制实验表明自由基中继机制负责级联反应。这种方法提供了一种有效且实用的方法来获得各种 1-perfluoroalkyl-3-heteroaryl 双环 [1.1.1] 戊烷 (BCP),并具有在有机合成中的各种应用潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号