当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cation-Coordinated Inner-Sphere CO2 Electroreduction at Au–Water Interfaces
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-01-11 , DOI: 10.1021/jacs.2c11643
Xueping Qin 1 , Tejs Vegge 1 , Heine Anton Hansen 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-01-11 , DOI: 10.1021/jacs.2c11643
Xueping Qin 1 , Tejs Vegge 1 , Heine Anton Hansen 1
Affiliation
|
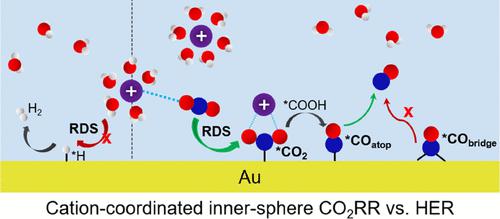
Electrochemical CO2 reduction reaction (CO2RR) is a promising technology for the clean energy economy. Numerous efforts have been devoted to enhancing the mechanistic understanding of CO2RR from both experimental and theoretical studies. Electrolyte ions are critical for the CO2RR; however, the role of alkali metal cations is highly controversial, and a complete free energy diagram of CO2RR at Au–water interfaces is still missing. Here, we provide a systematic mechanism study toward CO2RR via ab initio molecular dynamics simulations integrated with the slow-growth sampling (SG-AIMD) method. By using the SG-AIMD approach, we demonstrate that CO2RR is facile at the inner-sphere interface in the presence of K cations, which promote the CO2 activation with the free energy barrier of only 0.66 eV. Furthermore, the competitive hydrogen evolution reaction (HER) is inhibited by the interfacial cations with the induced kinetic blockage effect, where the rate-limiting Volmer step shows a much higher energy barrier (1.27 eV). Eventually, a comprehensive free energy diagram including both kinetics and thermodynamics of the CO2RR to CO and the HER at the electrochemical interface is derived, which illustrates the critical role of cations on the overall performance of CO2 electroreduction by facilitating CO2 adsorption while suppressing the hydrogen evolution at the same time.
中文翻译:

Au-水界面处的阳离子配位内球 CO2 电还原
电化学CO 2还原反应(CO 2 RR) 是一种有前途的清洁能源经济技术。许多努力已经致力于从实验和理论研究中提高对 CO 2 RR 的机理理解。电解质离子对 CO 2 RR 至关重要;然而,碱金属阳离子的作用存在很大争议,并且仍然缺少 Au-水界面处CO 2 RR 的完整自由能图。在这里,我们通过从头算分子动力学模拟与缓慢生长采样 (SG-AIMD) 方法相结合,对 CO 2 RR 进行了系统的机制研究。通过使用 SG-AIMD 方法,我们证明了 CO 2在存在 K 阳离子的情况下,RR 在球内界面处很容易,这促进了 CO 2的活化,自由能垒仅为 0.66 eV。此外,竞争性析氢反应 (HER) 受到界面阳离子的抑制,并具有诱导动力学阻塞效应,其中限速 Volmer 步骤显示出更高的能垒 (1.27 eV)。最终,得出了一个全面的自由能图,包括电化学界面处 CO 2 RR 到 CO 和 HER 的动力学和热力学,它说明了阳离子通过促进 CO 2吸附同时对 CO 2电还原的整体性能的关键作用同时抑制析氢。
更新日期:2023-01-11
中文翻译:

Au-水界面处的阳离子配位内球 CO2 电还原
电化学CO 2还原反应(CO 2 RR) 是一种有前途的清洁能源经济技术。许多努力已经致力于从实验和理论研究中提高对 CO 2 RR 的机理理解。电解质离子对 CO 2 RR 至关重要;然而,碱金属阳离子的作用存在很大争议,并且仍然缺少 Au-水界面处CO 2 RR 的完整自由能图。在这里,我们通过从头算分子动力学模拟与缓慢生长采样 (SG-AIMD) 方法相结合,对 CO 2 RR 进行了系统的机制研究。通过使用 SG-AIMD 方法,我们证明了 CO 2在存在 K 阳离子的情况下,RR 在球内界面处很容易,这促进了 CO 2的活化,自由能垒仅为 0.66 eV。此外,竞争性析氢反应 (HER) 受到界面阳离子的抑制,并具有诱导动力学阻塞效应,其中限速 Volmer 步骤显示出更高的能垒 (1.27 eV)。最终,得出了一个全面的自由能图,包括电化学界面处 CO 2 RR 到 CO 和 HER 的动力学和热力学,它说明了阳离子通过促进 CO 2吸附同时对 CO 2电还原的整体性能的关键作用同时抑制析氢。

































 京公网安备 11010802027423号
京公网安备 11010802027423号