当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biocatalytic Cascades toward Iminosugar Scaffolds Reveal Promiscuous Activity of Shikimate Dehydrogenases
ACS Central Science ( IF 12.7 ) Pub Date : 2023-01-11 , DOI: 10.1021/acscentsci.2c01169 Christopher R B Swanson 1 , Grayson J Ford 1 , Ashley P Mattey 1 , Léa Gourbeyre 1 , Sabine L Flitsch 1
ACS Central Science ( IF 12.7 ) Pub Date : 2023-01-11 , DOI: 10.1021/acscentsci.2c01169 Christopher R B Swanson 1 , Grayson J Ford 1 , Ashley P Mattey 1 , Léa Gourbeyre 1 , Sabine L Flitsch 1
Affiliation
|
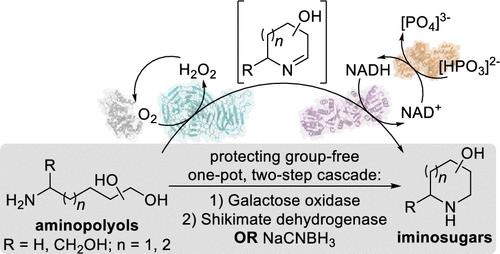
Iminosugar scaffolds are highly sought-after pharmaceutical targets, but their chemical synthesis is lengthy and can suffer from poor scalability and purification. Here we report protecting-group-free chemoenzymatic and biocatalytic cascades to synthesize iminosugars from sugar-derived aminopolyols in two steps. Using galactose oxidase variant F2 followed by a chemical or enzymatic reduction provided an efficient one-pot route to these targets, with product formation >70%. Key to success of this strategy was the application of genome mining, which identified bacterial shikimate dehydrogenases as promiscuous iminosugar reductases. The cell-free protocols allowed for isolation of highly polar iminosugar products from biotransformations in a single step through development of a gradient-elution cation exchange purification. The two-step pathway provides a short synthetic route that can be used as a cell-free platform for broader iminosugar synthesis.
中文翻译:

亚氨基糖支架的生物催化级联揭示莽草酸脱氢酶的混杂活性
亚氨基糖支架是备受追捧的药物靶标,但它们的化学合成过程冗长,而且可扩展性和纯化能力较差。在这里,我们报告了无保护基团的化学酶促和生物催化级联,分两步从糖衍生的氨基多元醇合成亚氨基糖。使用半乳糖氧化酶变体 F 2随后进行化学或酶促还原为这些目标提供了一种有效的一锅法途径,产物形成 >70%。该策略成功的关键是基因组挖掘的应用,它将细菌莽草酸脱氢酶鉴定为混杂的亚氨基糖还原酶。无细胞方案允许通过开发梯度洗脱阳离子交换纯化一步从生物转化中分离出高极性亚氨基糖产物。两步途径提供了一条较短的合成路线,可用作更广泛的亚氨基糖合成的无细胞平台。
更新日期:2023-01-11
中文翻译:

亚氨基糖支架的生物催化级联揭示莽草酸脱氢酶的混杂活性
亚氨基糖支架是备受追捧的药物靶标,但它们的化学合成过程冗长,而且可扩展性和纯化能力较差。在这里,我们报告了无保护基团的化学酶促和生物催化级联,分两步从糖衍生的氨基多元醇合成亚氨基糖。使用半乳糖氧化酶变体 F 2随后进行化学或酶促还原为这些目标提供了一种有效的一锅法途径,产物形成 >70%。该策略成功的关键是基因组挖掘的应用,它将细菌莽草酸脱氢酶鉴定为混杂的亚氨基糖还原酶。无细胞方案允许通过开发梯度洗脱阳离子交换纯化一步从生物转化中分离出高极性亚氨基糖产物。两步途径提供了一条较短的合成路线,可用作更广泛的亚氨基糖合成的无细胞平台。































 京公网安备 11010802027423号
京公网安备 11010802027423号