European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-01-11 , DOI: 10.1016/j.ejmech.2023.115114 Wei Ming 1 , Wen-Long Lu 1 , Christophe Pannecouque 2 , Jiong Chen 1 , Hai-Feng Wang 3 , Ya-Qi Xiao 1 , Sha Hu 1 , Shuang-Xi Gu 3 , Yuan-Yuan Zhu 4 , Fen-Er Chen 5
|
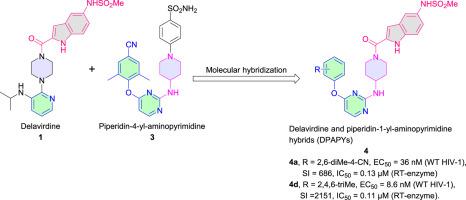
The hybrids of delavirdine and piperdin-4-yl-aminopyrimidine (DPAPYs) were designed from two excellent HIV-1 NNRTIs delavirdine and piperidin-4-yl-aminopyrimidine via molecular hybridization. The target compounds 4a-r were prepared and evaluated for their cellular anti-HIV activities and cytotoxicities as well as the inhibitory activities against HIV-1 reverse transcriptase (RT). All the newly synthesized compounds demonstrated moderate to excellent potency against wild-type (WT) HIV-1 with EC50 values in a range of 5.7 to 0.0086 μM and against RT with IC50 values ranging from 12.0 to 0.11 μM, indicating that the DPAPYs were specific RT inhibitors. Among all, 4d displayed the most potent activity against WT HIV-1 (EC50 = 8.6 nM, SI = 2151). Gratifyingly, it exhibited good to excellent potency against the single HIV-1 mutants L100I, K103N, Y181C, Y188L, E138K, as well as the double mutant F227L + V106A. Furthermore, the preliminary structure-activity relationships were summarized, molecular modeling was conducted to explore the binding mode of DPAPYs and HIV-1 RT, and their physicochemical properties were also predicted.
中文翻译:

地拉韦啶和哌啶-4-基-氨基嘧啶 (DPAPY) 的杂合体作为有效的 HIV-1 NNRTIs:设计、合成和生物活性
地拉韦啶和哌啶-4-基-氨基嘧啶(DPAPY)杂合体是由两种优良的HIV-1 NNRTIs地拉韦啶和哌啶-4-基-氨基嘧啶通过分子杂交设计而成。制备了目标化合物4a-r,并评估了其细胞抗HIV活性和细胞毒性以及对HIV-1逆转录酶(RT)的抑制活性。所有新合成的化合物均表现出中等至优异的抗野生型 (WT) HIV-1 效力,EC 50值在 5.7 至 0.0086 μM 范围内,而对抗 RT,IC 50值在 12.0 至 0.11 μM 范围内,表明 DPAPY是特异性 RT 抑制剂。其中,4d显示出针对 WT HIV-1 的最有效活性(EC 50 = 8.6 nM,SI = 2151)。令人欣慰的是,它对单一 HIV-1 突变体 L100I、K103N、Y181C、Y188L、E138K 以及双突变体 F227L + V106A 表现出良好至优异的效力。此外,还总结了初步的构效关系,并进行了分子建模来探索DPAPYs与HIV-1 RT的结合模式,并预测了它们的理化性质。

































 京公网安备 11010802027423号
京公网安备 11010802027423号