Synlett ( IF 1.7 ) Pub Date : 2023-01-10 , DOI: 10.1055/a-1982-5433 Nina gunawan 1 , michael Nutt 2 , Alex C. Bissember 3 , Jason Smith 1 , Scott Stewart 4
|
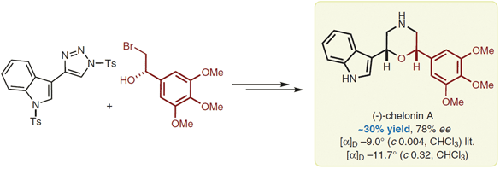
Substituted morpholine derivatives appear frequently in biologically active compounds and thus novel routes towards such structures are of great synthetic interest. Herein, we report the total syntheses of chelonin A, a morpholine-derived marine natural product with reported antibacterial and anti-inflammatory activity. The key step in this process was a rhodium carbenoid 1,3-insertion into a bromohydrin O–H bond, followed by annulation, leading to a 2,6-disubstituted-3,4-dihydro-2H-1,4-oxazine core. This work was then extended to deliver the first asymmetric total synthesis of (–)-chelonin A using an enantioenriched bromohydrin, prepared in turn via asymmetric transfer hydrogenation of an α-bromoketone.
中文翻译:

N-Tosyl-1,2,3-triazoles 作为吗啉的支架:(–)-Chelonin A 的全合成
取代的吗啉衍生物经常出现在生物活性化合物中,因此获得此类结构的新途径具有很大的合成意义。在此,我们报告了海龟素 A 的全合成,海龟素 A 是一种吗啉衍生的海洋天然产物,据报道具有抗菌和抗炎活性。该过程的关键步骤是将铑类胡萝卜素 1,3-插入溴醇 O-H 键,然后环化,生成 2,6-二取代-3,4-二氢-2 H -1,4-恶嗪核。然后将这项工作扩展到使用富含对映体的溴醇,通过 α-溴酮的不对称转移氢化依次制备 (–)-chelonin A 的第一个不对称全合成。































 京公网安备 11010802027423号
京公网安备 11010802027423号