当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Aerobic N-Nitrosation by Secondary Nitroalkanes in Water: A Tandem Diazotization of Aryl Amines and Azo Coupling
Organic Letters ( IF 4.9 ) Pub Date : 2023-01-10 , DOI: 10.1021/acs.orglett.2c04353 Karu Ramesh 1 , Hun Young Kim 2 , Kyungsoo Oh 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-01-10 , DOI: 10.1021/acs.orglett.2c04353 Karu Ramesh 1 , Hun Young Kim 2 , Kyungsoo Oh 1
Affiliation
|
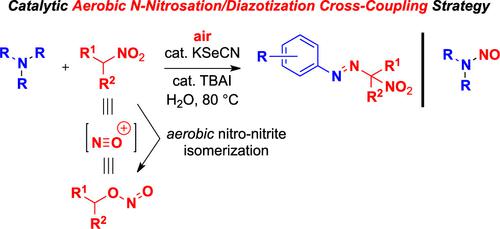
Secondary nitroalkanes underwent oxygen-mediated nitro–nitrite isomerization, serving as versatile N-nitrosating agents under aerobic conditions. To capitalize on the newly discovered aerobic nitro–nitrite isomerization phenomenon, a phase-transfer catalysis system employing KSeCN and TBAI was developed, in which the tandem diazotization and azo coupling with nitroalkanes as well as N-nitrosation of amines were accomplished. The current tandem diazotization and azo coupling strategy provides a facile synthesis of areneazo-2-(2-nitro)propane derivatives, a safe synthetic alternative to aryl diazonium salts.
中文翻译:

水中仲硝基烷烃催化好氧 N-亚硝化:芳胺和偶氮偶联的串联重氮化
仲硝基烷烃经历了氧介导的硝基-亚硝酸盐异构化,在有氧条件下用作多功能的 N-亚硝化剂。利用新发现的好氧硝基-亚硝酸盐异构化现象,开发了一种采用 KSeCN 和 TBAI 的相转移催化体系,实现了与硝基烷烃的串联重氮化和偶氮偶联以及胺的 N-亚硝化。当前的串联重氮化和偶氮偶联策略提供了芳基偶氮-2-(2-硝基)丙烷衍生物的简便合成,这是芳基重氮盐的安全合成替代品。
更新日期:2023-01-10
中文翻译:

水中仲硝基烷烃催化好氧 N-亚硝化:芳胺和偶氮偶联的串联重氮化
仲硝基烷烃经历了氧介导的硝基-亚硝酸盐异构化,在有氧条件下用作多功能的 N-亚硝化剂。利用新发现的好氧硝基-亚硝酸盐异构化现象,开发了一种采用 KSeCN 和 TBAI 的相转移催化体系,实现了与硝基烷烃的串联重氮化和偶氮偶联以及胺的 N-亚硝化。当前的串联重氮化和偶氮偶联策略提供了芳基偶氮-2-(2-硝基)丙烷衍生物的简便合成,这是芳基重氮盐的安全合成替代品。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号