当前位置:
X-MOL 学术
›
Bull. Korean Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of five-ring-fused azepinone derivatives from bis-isatins via sequential 6π-electrocyclic ring closure, ring expansion, and dehydrogenation process
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2023-01-08 , DOI: 10.1002/bkcs.12672 Sangku Lee 1 , Junseong Lee 2 , Jae Nyoung Kim 2
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2023-01-08 , DOI: 10.1002/bkcs.12672 Sangku Lee 1 , Junseong Lee 2 , Jae Nyoung Kim 2
Affiliation
|
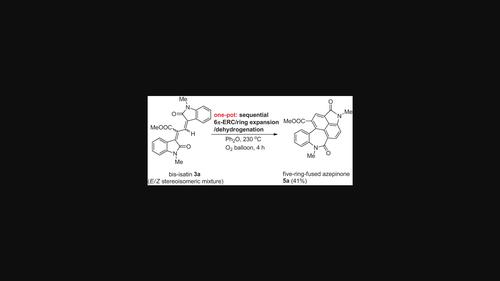
Five-ring-fused azepinone derivatives were synthesized serendipitously from bis-isatins via sequential 6π-electrocyclic ring closure, ring expansion, and dehydrogenation process in moderate yields (34%–47%). The reaction was carried out in diphenyl ether at 230 °C for 4–12 h under O2 balloon atmosphere.
中文翻译:

通过顺序 6π-电环闭环、扩环和脱氢过程从双靛红合成五环稠合氮杂庚酮衍生物
通过顺序 6π-电环闭环、扩环和脱氢过程,以中等产率 (34%–47%) 从双靛红偶然合成了五环稠合氮杂庚酮衍生物。反应在二苯醚中于 230 °C 在 O 2气球气氛下进行 4-12 小时。
更新日期:2023-01-08
中文翻译:

通过顺序 6π-电环闭环、扩环和脱氢过程从双靛红合成五环稠合氮杂庚酮衍生物
通过顺序 6π-电环闭环、扩环和脱氢过程,以中等产率 (34%–47%) 从双靛红偶然合成了五环稠合氮杂庚酮衍生物。反应在二苯醚中于 230 °C 在 O 2气球气氛下进行 4-12 小时。






























 京公网安备 11010802027423号
京公网安备 11010802027423号