当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lattice oxygen activation in disordered rocksalts for boosting oxygen evolution
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2023-01-10 , DOI: 10.1039/d2cp05531g Menghan Zhao 1 , Xuerong Zheng 1, 2 , Chengchi Cao 2 , Qi Lu 1 , Jinfeng Zhang 1 , Haozhi Wang 2 , Zhong Huang 2 , Yanhui Cao 1 , Yang Wang 2 , Yida Deng 1, 2
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2023-01-10 , DOI: 10.1039/d2cp05531g Menghan Zhao 1 , Xuerong Zheng 1, 2 , Chengchi Cao 2 , Qi Lu 1 , Jinfeng Zhang 1 , Haozhi Wang 2 , Zhong Huang 2 , Yanhui Cao 1 , Yang Wang 2 , Yida Deng 1, 2
Affiliation
|
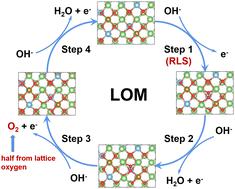
The recent development of some special oxygen evolution reaction (OER) electrocatalysts shows that the lattice oxygen could participate in the catalysis process via the lattice oxygen oxidation mechanism (LOM), which the provides good possibility of exploring advanced electrocatalysts that could overcome the scaling relationship in conventional catalysis processes through a traditional adsorbate evolution mechanism. In this work, we theoretically predict that, benefiting from the unhybridized O–Li orbitals and the resulting metastable Li–O–Li ligands, the lattice oxygen could be easily activated and oxidized at relatively high oxidation voltages. Thus, lithium-excess disordered rocksalts (DRX) should possess the potential for acting as active OER electrocatalysts, which catalyze through the LOM pathway. The isotope labelling experimental results show that the lattice oxygen in the DRX was activated and participated in the OER process through the LOM pathway. The typical DRX of Li1.2Fe0.4Ti0.5O2 displays obviously pH-dependent OER activity under the LOM process and shows a low overpotential of 263 mV to reach 10 mA cm−2 with long-term stability for 100 hours. The turnover frequency of Li1.2Fe0.4Ti0.5O2 is nearly 9 times that of LiFePO4 at the overpotential of 300 mV. This work opens a new chemical space for exploring efficient electrocatalysts to enhance the OER performance through the LOM pathway.
中文翻译:

无序岩盐中的晶格氧活化促进氧气释放
最近开发的一些特殊的析氧反应 (OER) 电催化剂表明,晶格氧可以通过以下方式参与催化过程晶格氧氧化机制(LOM),这为探索先进的电催化剂提供了很好的可能性,这些电催化剂可以通过传统的吸附物演化机制克服传统催化过程中的比例关系。在这项工作中,我们从理论上预测,受益于未杂化的 O-Li 轨道和由此产生的亚稳态 Li-O-Li 配体,晶格氧可以在相对高的氧化电压下容易地被激活和氧化。因此,锂过量的无序岩盐 (DRX) 应该具有作为活性 OER 电催化剂的潜力,它通过 LOM 途径进行催化。同位素标记实验结果表明,DRX中的晶格氧被激活并通过LOM途径参与OER过程。Li 1.2的典型DRXFe 0.4 Ti 0.5 O 2在LOM过程下表现出明显的pH依赖性OER活性,并表现出263 mV的低过电势以达到10 mA cm -2并具有100小时的长期稳定性。在300 mV的过电位下,Li 1.2 Fe 0.4 Ti 0.5 O 2的周转频率是LiFePO 4的近9倍。这项工作为探索高效电催化剂以通过 LOM 途径提高 OER 性能开辟了一个新的化学空间。
更新日期:2023-01-10
中文翻译:

无序岩盐中的晶格氧活化促进氧气释放
最近开发的一些特殊的析氧反应 (OER) 电催化剂表明,晶格氧可以通过以下方式参与催化过程晶格氧氧化机制(LOM),这为探索先进的电催化剂提供了很好的可能性,这些电催化剂可以通过传统的吸附物演化机制克服传统催化过程中的比例关系。在这项工作中,我们从理论上预测,受益于未杂化的 O-Li 轨道和由此产生的亚稳态 Li-O-Li 配体,晶格氧可以在相对高的氧化电压下容易地被激活和氧化。因此,锂过量的无序岩盐 (DRX) 应该具有作为活性 OER 电催化剂的潜力,它通过 LOM 途径进行催化。同位素标记实验结果表明,DRX中的晶格氧被激活并通过LOM途径参与OER过程。Li 1.2的典型DRXFe 0.4 Ti 0.5 O 2在LOM过程下表现出明显的pH依赖性OER活性,并表现出263 mV的低过电势以达到10 mA cm -2并具有100小时的长期稳定性。在300 mV的过电位下,Li 1.2 Fe 0.4 Ti 0.5 O 2的周转频率是LiFePO 4的近9倍。这项工作为探索高效电催化剂以通过 LOM 途径提高 OER 性能开辟了一个新的化学空间。































 京公网安备 11010802027423号
京公网安备 11010802027423号