当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Assembly Mitochondria-Targeting Donor–Acceptor Type Theranostic Nanosphere Activates ROS Storm for Multimodal Cancer Therapy
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2023-01-10 , DOI: 10.1021/acsabm.2c00942 Wen-Juan Gao 1 , Meng-Meng Wang 1 , Yan Su 1, 2 , Zheng-Hong Yu 2 , Hong-Ke Liu 1 , Zhi Su 1
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2023-01-10 , DOI: 10.1021/acsabm.2c00942 Wen-Juan Gao 1 , Meng-Meng Wang 1 , Yan Su 1, 2 , Zheng-Hong Yu 2 , Hong-Ke Liu 1 , Zhi Su 1
Affiliation
|
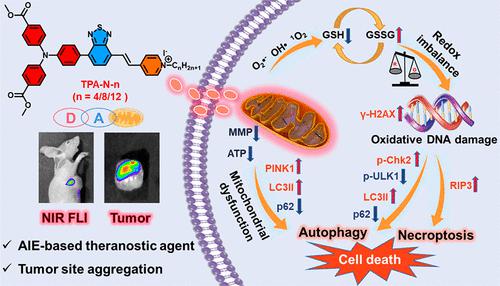
The rational design of cancer theranostics with natural diagnostic information and therapeutic behavior has been considered to be a big challenge, since common theranostics from photothermal and photodynamic therapy need to be activated with external stimuli of photoirradiation to enable the chemotherapeutic effects. In this contribution, we have designed and synthesized a series of simple theranostic agents, TPA-N-n (n = 4, 8, 12), which could accumulate at the tumor site over 48 h and indicate superior antiproliferative performance in vivo. TPA-N-n was constructed with electron donor triphenylamine-acceptor benzothiadiazole-mitochondria-targeting moiety pyridinium. Complex TPA-N-8 indicated the best cytotoxicity to cancerous HeLa cells, with an IC50 value of 4.3 μM, and could self-assemble to a nanosphere with a size of 161.2 nm in the DMSO/PBS solution. It is worth noting that TPA-N-8 could accumulate in the mitochondria and produce major ROS species O2•– and OH• as well as small amounts of 1O2 without photoirradiation. Oxidative DNA damage is initiated due to the imbalance of intracellular redox homeostasis from the significant ROS storm. Multimodal synergistic therapy for HeLa cells was activated, as the PINK1-mediated mitophagy from the damaged mitochondria and DNA damage responsive (DDR) induced necroptosis and autophagy. This work not only provided a successful D–A type theranostic agent with superior anticancer performance from multimodal synergistic therapy but also further demonstrated the high efficacy of a mitochondria-targeting strategy for cancer treatment.
中文翻译:

自组装线粒体靶向供体-受体型治疗诊断纳米球激活 ROS 风暴用于多模式癌症治疗
合理设计具有自然诊断信息和治疗行为的癌症治疗诊断被认为是一个巨大的挑战,因为来自光热和光动力疗法的常见治疗诊断需要用光照射的外部刺激来激活以实现化学治疗效果。在此贡献中,我们设计并合成了一系列简单的治疗诊断剂TPA-N- n(n = 4、8、12),它们可以在肿瘤部位积聚超过 48 小时,并表明在体内具有优异的抗增殖性能。TPA-N- n由电子供体三苯胺-受体苯并噻二唑-线粒体-靶向部分吡啶构建。复杂的TPA-N-8表明对癌细胞HeLa细胞的细胞毒性最好,IC 50值为4.3 μM,并且可以在DMSO/PBS溶液中自组装成尺寸为161.2 nm的纳米球。值得注意的是,TPA-N-8可在线粒体中积累并产生主要的 ROS 种类 O 2 • -和 OH• 以及少量的1 O 2没有光照射。由于显着的 ROS 风暴导致细胞内氧化还原稳态失衡,导致 DNA 氧化损伤。HeLa 细胞的多模式协同治疗被激活,因为 PINK1 介导的来自受损线粒体的线粒体自噬和 DNA 损伤反应 (DDR) 诱导坏死和自噬。这项工作不仅提供了一种成功的 D-A 型治疗诊断剂,具有来自多模式协同疗法的卓越抗癌性能,而且进一步证明了线粒体靶向策略在癌症治疗中的高效性。
更新日期:2023-01-10
中文翻译:

自组装线粒体靶向供体-受体型治疗诊断纳米球激活 ROS 风暴用于多模式癌症治疗
合理设计具有自然诊断信息和治疗行为的癌症治疗诊断被认为是一个巨大的挑战,因为来自光热和光动力疗法的常见治疗诊断需要用光照射的外部刺激来激活以实现化学治疗效果。在此贡献中,我们设计并合成了一系列简单的治疗诊断剂TPA-N- n(n = 4、8、12),它们可以在肿瘤部位积聚超过 48 小时,并表明在体内具有优异的抗增殖性能。TPA-N- n由电子供体三苯胺-受体苯并噻二唑-线粒体-靶向部分吡啶构建。复杂的TPA-N-8表明对癌细胞HeLa细胞的细胞毒性最好,IC 50值为4.3 μM,并且可以在DMSO/PBS溶液中自组装成尺寸为161.2 nm的纳米球。值得注意的是,TPA-N-8可在线粒体中积累并产生主要的 ROS 种类 O 2 • -和 OH• 以及少量的1 O 2没有光照射。由于显着的 ROS 风暴导致细胞内氧化还原稳态失衡,导致 DNA 氧化损伤。HeLa 细胞的多模式协同治疗被激活,因为 PINK1 介导的来自受损线粒体的线粒体自噬和 DNA 损伤反应 (DDR) 诱导坏死和自噬。这项工作不仅提供了一种成功的 D-A 型治疗诊断剂,具有来自多模式协同疗法的卓越抗癌性能,而且进一步证明了线粒体靶向策略在癌症治疗中的高效性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号