当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
AlphaFold accelerates artificial intelligence powered drug discovery: efficient discovery of a novel CDK20 small molecule inhibitor
Chemical Science ( IF 7.6 ) Pub Date : 2023-01-10 , DOI: 10.1039/d2sc05709c Feng Ren 1 , Xiao Ding 1 , Min Zheng 1 , Mikhail Korzinkin 2 , Xin Cai 1 , Wei Zhu 1 , Alexey Mantsyzov 2 , Alex Aliper 2 , Vladimir Aladinskiy 2 , Zhongying Cao 1 , Shanshan Kong 1 , Xi Long 2 , Bonnie Hei Man Liu 2 , Yingtao Liu 1 , Vladimir Naumov 2 , Anastasia Shneyderman 2 , Ivan V Ozerov 2 , Ju Wang 1 , Frank W Pun 2 , Daniil A Polykovskiy 2 , Chong Sun 3 , Michael Levitt 4 , Alán Aspuru-Guzik 3 , Alex Zhavoronkov 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2023-01-10 , DOI: 10.1039/d2sc05709c Feng Ren 1 , Xiao Ding 1 , Min Zheng 1 , Mikhail Korzinkin 2 , Xin Cai 1 , Wei Zhu 1 , Alexey Mantsyzov 2 , Alex Aliper 2 , Vladimir Aladinskiy 2 , Zhongying Cao 1 , Shanshan Kong 1 , Xi Long 2 , Bonnie Hei Man Liu 2 , Yingtao Liu 1 , Vladimir Naumov 2 , Anastasia Shneyderman 2 , Ivan V Ozerov 2 , Ju Wang 1 , Frank W Pun 2 , Daniil A Polykovskiy 2 , Chong Sun 3 , Michael Levitt 4 , Alán Aspuru-Guzik 3 , Alex Zhavoronkov 1, 2
Affiliation
|
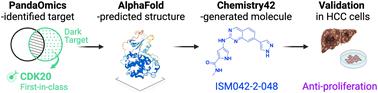
The application of artificial intelligence (AI) has been considered a revolutionary change in drug discovery and development. In 2020, the AlphaFold computer program predicted protein structures for the whole human genome, which has been considered a remarkable breakthrough in both AI applications and structural biology. Despite the varying confidence levels, these predicted structures could still significantly contribute to structure-based drug design of novel targets, especially the ones with no or limited structural information. In this work, we successfully applied AlphaFold to our end-to-end AI-powered drug discovery engines, including a biocomputational platform PandaOmics and a generative chemistry platform Chemistry42. A novel hit molecule against a novel target without an experimental structure was identified, starting from target selection towards hit identification, in a cost- and time-efficient manner. PandaOmics provided the protein of interest for the treatment of hepatocellular carcinoma (HCC) and Chemistry42 generated the molecules based on the structure predicted by AlphaFold, and the selected molecules were synthesized and tested in biological assays. Through this approach, we identified a small molecule hit compound for cyclin-dependent kinase 20 (CDK20) with a binding constant Kd value of 9.2 ± 0.5 μM (n = 3) within 30 days from target selection and after only synthesizing 7 compounds. Based on the available data, a second round of AI-powered compound generation was conducted and through this, a more potent hit molecule, ISM042-2-048, was discovered with an average Kd value of 566.7 ± 256.2 nM (n = 3). Compound ISM042-2-048 also showed good CDK20 inhibitory activity with an IC50 value of 33.4 ± 22.6 nM (n = 3). In addition, ISM042-2-048 demonstrated selective anti-proliferation activity in an HCC cell line with CDK20 overexpression, Huh7, with an IC50 of 208.7 ± 3.3 nM, compared to a counter screen cell line HEK293 (IC50 = 1706.7 ± 670.0 nM). This work is the first demonstration of applying AlphaFold to the hit identification process in drug discovery.
中文翻译:

AlphaFold加速人工智能驱动的药物发现:高效发现新型CDK20小分子抑制剂
人工智能(AI)的应用被认为是药物发现和开发的革命性变化。2020年,AlphaFold计算机程序预测了整个人类基因组的蛋白质结构,这被认为是人工智能应用和结构生物学方面的重大突破。尽管置信水平不同,这些预测的结构仍然可以为新靶标的基于结构的药物设计做出重大贡献,特别是那些没有或有限结构信息的靶标。在这项工作中,我们成功地将 AlphaFold 应用于端到端人工智能驱动的药物发现引擎,包括生物计算平台 PandaOmics 和生成化学平台 Chemistry42。在没有实验结构的情况下,鉴定出一种针对新靶点的新型命中分子,从靶点选择到命中识别,以一种经济高效的方式。PandaOmics 提供了用于治疗肝细胞癌 (HCC) 的目标蛋白质,Chemistry42 根据 AlphaFold 预测的结构生成了分子,并合成了选定的分子并在生物测定中进行了测试。通过这种方法,我们在靶点选择后 30 天内仅合成了 7 种化合物,就鉴定出了一种针对细胞周期蛋白依赖性激酶 20 (CDK20) 的小分子命中化合物,其结合常数 Kd 值为 9.2 ± 0.5 μM ( n = 3)。根据现有数据,进行了第二轮人工智能驱动的化合物生成,通过这一过程,发现了更有效的命中分子 ISM042-2-048,其平均 Kd 值为 566.7 ± 256.2 nM ( n = 3) 。化合物ISM042-2-048还表现出良好的CDK20抑制活性,IC 50值为33.4 ± 22.6 nM ( n = 3)。此外,与计数器筛选细胞系 HEK293 相比,ISM042-2-048 在 CDK20 过表达的 HCC 细胞系 Huh7 中表现出选择性抗增殖活性, IC 50为 208.7 ± 3.3 nM(IC 50 = 1706.7 ± 670.0纳米)。这项工作是首次演示将 AlphaFold 应用到药物发现的命中识别过程中。
更新日期:2023-01-10
中文翻译:

AlphaFold加速人工智能驱动的药物发现:高效发现新型CDK20小分子抑制剂
人工智能(AI)的应用被认为是药物发现和开发的革命性变化。2020年,AlphaFold计算机程序预测了整个人类基因组的蛋白质结构,这被认为是人工智能应用和结构生物学方面的重大突破。尽管置信水平不同,这些预测的结构仍然可以为新靶标的基于结构的药物设计做出重大贡献,特别是那些没有或有限结构信息的靶标。在这项工作中,我们成功地将 AlphaFold 应用于端到端人工智能驱动的药物发现引擎,包括生物计算平台 PandaOmics 和生成化学平台 Chemistry42。在没有实验结构的情况下,鉴定出一种针对新靶点的新型命中分子,从靶点选择到命中识别,以一种经济高效的方式。PandaOmics 提供了用于治疗肝细胞癌 (HCC) 的目标蛋白质,Chemistry42 根据 AlphaFold 预测的结构生成了分子,并合成了选定的分子并在生物测定中进行了测试。通过这种方法,我们在靶点选择后 30 天内仅合成了 7 种化合物,就鉴定出了一种针对细胞周期蛋白依赖性激酶 20 (CDK20) 的小分子命中化合物,其结合常数 Kd 值为 9.2 ± 0.5 μM ( n = 3)。根据现有数据,进行了第二轮人工智能驱动的化合物生成,通过这一过程,发现了更有效的命中分子 ISM042-2-048,其平均 Kd 值为 566.7 ± 256.2 nM ( n = 3) 。化合物ISM042-2-048还表现出良好的CDK20抑制活性,IC 50值为33.4 ± 22.6 nM ( n = 3)。此外,与计数器筛选细胞系 HEK293 相比,ISM042-2-048 在 CDK20 过表达的 HCC 细胞系 Huh7 中表现出选择性抗增殖活性, IC 50为 208.7 ± 3.3 nM(IC 50 = 1706.7 ± 670.0纳米)。这项工作是首次演示将 AlphaFold 应用到药物发现的命中识别过程中。































 京公网安备 11010802027423号
京公网安备 11010802027423号