Resources, Conservation and Recycling ( IF 11.2 ) Pub Date : 2023-01-09 , DOI: 10.1016/j.resconrec.2022.106857
Ruizhao Yan , Bang Li , Mingxian Zhou , Jia Li , Zhenming Xu
|
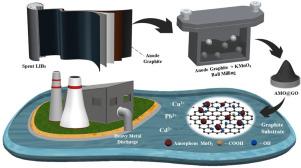
Resource recycling has taken center stage of global carbon neutrality. In this study, we reclaimed spent graphite (SG) from retired lithium-ion batteries (LIBs) and introduced potassium permanganate powder to prepare amorphous MnO2 loaded graphite oxide (AMO@GO) via one-step mechanochemical method for heavy metals adsorption. The synthesized AMO@GO was characterized by X-ray diffraction (XRD), Raman spectra, X-ray Photoelectron Spectroscopy (XPS), Brunauer-Emmett-Teller Method (BET), Fourier Transform Infrared Spectrometer (FTIR), and Scanning Electron Microscope (SEM), which illustrated AMO@GO had richer pore structure, ample active groups, strong surface ion exchange activity. These properties made AMO@GO a highly potential adsorbent for aqueous heavy metal contamination. The results showed the adsorption capacities of AMO@GO for Cu2+, Pb2+ and Cd2+ in the water body can reach 233.99 mg/g, 353.13 mg/g and 257.95 mg/g respectively (calculated by Langmuir isotherm model), significantly higher than similar graphite-based adsorbents. Through in-depth studies of the adsorption mechanism, we found that the ion exchange of heavy metals with active groups on the surface of graphite oxide and amorphous MnO2 was the main source of sample adsorption capacity.
中文翻译:

废锂离子电池正极石墨一步法机械化学高效合成重金属吸附剂
资源回收已成为全球碳中和的中心舞台。在这项研究中,我们从退役锂离子电池(LIBs)中回收废石墨(SG)并引入高锰酸钾粉末制备非晶MnO 2通过一步机械化学法负载氧化石墨 (AMO@GO) 吸附重金属。通过 X 射线衍射 (XRD)、拉曼光谱、X 射线光电子能谱 (XPS)、Brunauer-Emmett-Teller 方法 (BET)、傅里叶变换红外光谱仪 (FTIR) 和扫描电子显微镜对合成的 AMO@GO 进行了表征(SEM),表明AMO@GO具有更丰富的孔结构、丰富的活性基团、强的表面离子交换活性。这些特性使 AMO@GO 成为一种极具潜力的水性重金属污染吸附剂。结果表明AMO@GO对Cu 2+、Pb 2+和Cd 2+的吸附能力在水体中可分别达到233.99 mg/g、353.13 mg/g和257.95 mg/g(按Langmuir等温线模型计算),明显高于同类石墨基吸附剂。通过对其吸附机理的深入研究,我们发现氧化石墨和非晶态MnO 2表面活性基团对重金属的离子交换是样品吸附能力的主要来源。

































 京公网安备 11010802027423号
京公网安备 11010802027423号