当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing Electrocatalytic Hydrodechlorination through Interfacial Microenvironment Modulation
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-01-08 , DOI: 10.1021/acs.est.2c07462 Zhimin Fan 1, 2, 3 , Huachao Zhao 2, 3 , Kaifeng Wang 2, 3 , Wei Ran 2 , Jie-Fang Sun 4 , Jingfu Liu 1, 2, 3 , Rui Liu 1, 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-01-08 , DOI: 10.1021/acs.est.2c07462 Zhimin Fan 1, 2, 3 , Huachao Zhao 2, 3 , Kaifeng Wang 2, 3 , Wei Ran 2 , Jie-Fang Sun 4 , Jingfu Liu 1, 2, 3 , Rui Liu 1, 2
Affiliation
|
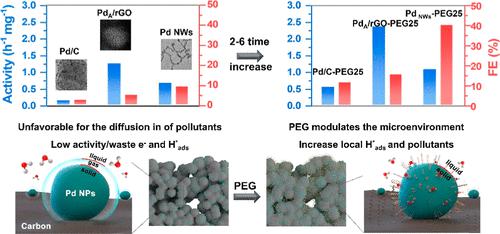
Electrochemical reduction (ER) is a promising approach to safely remove pollutants. However, sluggish reaction kinetics and significant side reactions considerably limit the applicability of this green process. Herein, we uncovered the previously ignored role of interfacial hydrophilicity in determining the ER performance through electron microscopy observations, contact angle (CA) analysis, and electrochemical measurements. A Pd/C electrocatalyst forms dense nanopores on the electrode surface, rendering it highly hydrophobic and achieving a CA of up to 145°. This imposes a large mass-transfer barrier for the diffusion of water and pollutants into Pd sites. Moreover, the release of H2 is suppressed, which changes the solid–liquid (Pd–polluted water) interface into a solid–gas (H2)–liquid interface. This further slows down mass transfer and the decontamination process. This dilemma can be easily alleviated by adding hydrophilic polymers like polyethylene glycol to increase hydrophilicity and improve mass transfer. By this way, the activity and Faraday efficiency of Pd/C in the electrochemical hydrodehalogenation of 2,4-dichlorophenol could be increased by 4–5 times. Moreover, this interfacial microenvironment modulation strategy is parallel to other approaches, such as Pd structural engineering, and therefore these strategies can be combined to further increase the electrochemical decontamination performance of electrocatalysts.
中文翻译:

通过界面微环境调节增强电催化加氢脱氯
电化学还原(ER)是安全去除污染物的一种有前景的方法。然而,缓慢的反应动力学和显着的副反应极大地限制了这种绿色工艺的适用性。在此,我们通过电子显微镜观察、接触角 (CA) 分析和电化学测量,发现了之前被忽视的界面亲水性在确定 ER 性能方面的作用。 Pd/C 电催化剂在电极表面形成致密的纳米孔,使其具有高度疏水性,并实现高达 145° 的 CA。这对水和污染物扩散到 Pd 位点施加了巨大的传质障碍。此外,H 2的释放受到抑制,从而将固-液(Pd-污染水)界面转变为固-气(H 2 )-液界面。这进一步减慢了传质和净化过程。通过添加聚乙二醇等亲水性聚合物来增加亲水性并改善传质,可以轻松缓解这种困境。这样,Pd/C在2,4-二氯苯酚电化学加氢脱卤反应中的活性和法拉第效率可提高4~5倍。此外,这种界面微环境调节策略与其他方法(例如Pd结构工程)是并行的,因此可以将这些策略结合起来,以进一步提高电催化剂的电化学去污性能。
更新日期:2023-01-08
中文翻译:

通过界面微环境调节增强电催化加氢脱氯
电化学还原(ER)是安全去除污染物的一种有前景的方法。然而,缓慢的反应动力学和显着的副反应极大地限制了这种绿色工艺的适用性。在此,我们通过电子显微镜观察、接触角 (CA) 分析和电化学测量,发现了之前被忽视的界面亲水性在确定 ER 性能方面的作用。 Pd/C 电催化剂在电极表面形成致密的纳米孔,使其具有高度疏水性,并实现高达 145° 的 CA。这对水和污染物扩散到 Pd 位点施加了巨大的传质障碍。此外,H 2的释放受到抑制,从而将固-液(Pd-污染水)界面转变为固-气(H 2 )-液界面。这进一步减慢了传质和净化过程。通过添加聚乙二醇等亲水性聚合物来增加亲水性并改善传质,可以轻松缓解这种困境。这样,Pd/C在2,4-二氯苯酚电化学加氢脱卤反应中的活性和法拉第效率可提高4~5倍。此外,这种界面微环境调节策略与其他方法(例如Pd结构工程)是并行的,因此可以将这些策略结合起来,以进一步提高电催化剂的电化学去污性能。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号