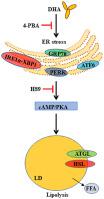
二十二碳六烯酸 (DHA) 是一种具有生物活性的脂肪酸,可减少脂质的积累。然而,这一过程背后的分子机制,尤其是在鱼类中,尚不清楚。最近的研究表明,内质网 (ER) 应激会触发未折叠蛋白反应的激活,这已被证明在脂质代谢中起着重要作用。在这项研究中,我们探讨了 DHA 对内质网应激的影响,并研究了 DHA 诱导草鱼脂肪细胞脂肪分解的潜在分子机制(Ctenopharyngodon idella) 在体内和体外。我们发现 DHA 显着降低甘油三酯含量,增加甘油分泌,促进脂肪细胞脂肪分解并诱发内质网应激,而使用 4-苯基丁酸 (4-PBA) 抑制内质网应激会抑制 DHA 的作用( P < 0.05 ) . 这些结果暗示 ER 应激可能参与 DHA 诱导的脂肪细胞脂肪分解。此外,STF-083010 是一种特定的肌醇需要酶 1α (IRE1α) 抑制剂,可减弱 DHA 对脂肪分解的影响,表明 IRE1α 和 X-box 结合蛋白 1 可能参与 DHA 诱导的脂肪分解。DHA 还通过增加 cAMP 水平和激活 PKA 酶来激活环磷酸腺苷 (cAMP) 依赖性蛋白激酶 A (PKA) 通路(P < 0.05)。尽管如此,PKA 抑制剂 H89 通过抑制 cAMP/PKA 信号通路削弱了 DHA 诱导的脂解作用。此外,使用 4-PBA 抑制内质网应激也能抑制脂肪分解并减轻 DHA 诱导的 cAMP/PKA 信号通路激活,表明内质网应激可能通过激活 cAMP/PKA 信号通路参与 DHA 诱导的脂肪分解。我们的数据表明,补充 DHA 可能是改善草鱼脂质积累的一种很有前途的营养策略。本研究阐明了 DHA 诱导草鱼脂肪细胞脂肪分解的分子机制,并强调了内质网应激和 cAMP/PKA 通路在 DHA 诱导脂肪分解中的重要性。这些结果加深了我们对通过靶向 DHA 改善淡水鱼脂质沉积的理解。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
DHA induces adipocyte lipolysis through endoplasmic reticulum stress and the cAMP/PKA signaling pathway in grass carp (Ctenopharyngodon idella)
Docosahexaenoic acid (DHA) is a biologically active fatty acid that reduces the accumulation of lipids. However, the molecular mechanism underlying this process, particularly in fish, is not well understood. Recent studies show that endoplasmic reticulum (ER) stress triggers the activation of the unfolded protein response, which has been revealed to play an essential role in lipid metabolism. In this study, we explored the effect of DHA on ER stress and investigated the potential molecular mechanisms underlying DHA-induced adipocyte lipolysis in grass carp (Ctenopharyngodon idella) both in vivo and in vitro. We found that DHA remarkably reduced the triglyceride content, increased the secretion of glycerol, promoted lipolysis in adipocytes and evoked ER stress, whereas inhibiting ER stress using 4-phenyl butyric acid (4-PBA) inhibited the effects of DHA (P < 0.05). These results implied that ER stress potentially participates in DHA-induced adipocyte lipolysis. Additionally, STF-083010, a specific inositol-requiring enzyme 1α (IRE1α)-inhibitor, attenuated the effects of DHA on lipolysis, demonstrating that IRE1α and X-box binding protein 1 potentially participate in DHA-induced lipolysis. DHA also activated the cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) pathway by increasing the level of cAMP and activating the PKA enzyme (P < 0.05). Nevertheless, H89, a PKA inhibitor, weakened DHA-induced lipolysis by inhibiting the cAMP/PKA signaling pathway. Furthermore, inhibiting ER stress using 4-PBA also inhibited lipolysis and alleviated DHA-induced activation of the cAMP/PKA signaling pathway, suggesting that ER stress may participate in DHA-induced lipolysis through the activation of the cAMP/PKA signaling pathway. Our data illustrate that DHA supplementation can be a promising nutritional strategy for ameliorating lipid accumulation in grass carp. The present study elucidated the molecular mechanism for DHA-induced lipolysis in grass carp adipocytes and emphasized the importance of ER stress and the cAMP/PKA pathway in DHA-induced lipolysis. These results deepen our understanding of ameliorating lipids deposition in freshwater fish by targeting DHA.



















































 京公网安备 11010802027423号
京公网安备 11010802027423号