Energy Storage Materials ( IF 18.9 ) Pub Date : 2023-01-07 , DOI: 10.1016/j.ensm.2023.01.009 J. Mark Weller , Minyuan M. Li , Evgueni Polikarpov , Kee Sung Han , Neil Kidner , Anant Patel , Mai Nguyen , Meghan Stout , Michael Gossett , Keeyoung Jung , David M. Reed , Vincent L. Sprenkle , Guosheng Li
|
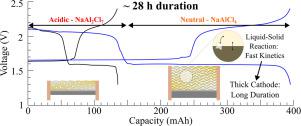
Sodium and aluminum are a natural combination of inexpensive, abundant elements as a redox pair for battery energy storage. Recent explorations pairing a sodium anode and aluminum cathode have demonstrated reversible, energy dense Na-Al cells with excellent rate capability using the electrochemical reaction between a molten Na anode and a NaAlCl4/Al cathode. In this work, the NaAlCl4/Al cathode is extended beyond the neutral NaAlCl4 composition by unlocking the NaCl-AlCl3 phase diagram to explore the extra accessible capacity hidden in acidic chloroaluminate melts up to and beyond the composition NaAl2Cl7. This enables higher specific capacity and average discharge voltages than previous Na-Al batteries, utilizing two distinct cell reaction mechanisms in one battery. Fundamental aspects of the NaAlCl4-NaAl2Cl7 reaction chemistry are investigated, and Na-metal/chloroaluminate batteries with excellent reversibility and areal capacity are demonstrated. Increasing the voltage window of the chloroaluminate Na-Al battery takes advantage of the higher voltage (∼ 2 V vs ∼1.6 V for neutral NaAlCl4) contributed by the acidic chloroaluminate cathode reaction, unlocking an additional specific energy of ∼119 Wh kg−1 by utilizing the conversion of NaAlCl4 to NaAl2Cl7, which adds to the neutral melt reaction between NaAlCl4/Al and Na (∼493 Wh kg−1 theoretical). By significantly increasing the cathode thickness and therefore accessible areal capacity up to 131.7 mAh cm−2, a discharge duration of 28.2 h is achieved with an estimated raw active materials cost of $7.02 kWh−1. These metrics show the great potential of this unlocked chloroaluminate battery for future low-cost, long-duration electrochemical energy storage.
中文翻译:

解锁低成本、长寿命 Na-Al 电池的 NaCl-AlCl3 相图
钠和铝是廉价、丰富元素的天然组合,作为电池储能的氧化还原对。最近将钠阳极和铝阴极配对的探索表明,利用熔融钠阳极和 NaAlCl 4 /Al 阴极之间的电化学反应,可逆、能量密集的 Na-Al 电池具有出色的倍率性能。在这项工作中,NaAlCl 4 /Al 阴极通过解锁 NaCl-AlCl 3相图扩展到中性 NaAlCl 4成分之外,以探索隐藏在酸性氯铝酸盐熔体中的额外可访问容量,直至和超过成分 NaAl 2 Cl 7. 这使得比以前的 Na-Al 电池具有更高的比容量和平均放电电压,在一个电池中利用两种不同的电池反应机制。研究了 NaAlCl 4 -NaAl 2 Cl 7反应化学的基本方面,并展示了具有出色可逆性和面积容量的钠金属/氯铝酸盐电池。增加氯铝酸盐 Na-Al 电池的电压窗口利用了酸性氯铝酸盐阴极反应提供的更高电压(∼ 2 V vs ∼1.6 V 中性 NaAlCl 4),释放了∼119 Wh kg −1的额外比能通过利用 NaAlCl 4到 NaAl 2 Cl的转化7,它增加了 NaAlCl 4 /Al 和 Na 之间的中性熔融反应(~493 Wh kg -1理论值)。通过显着增加阴极厚度并因此将可及面积容量增加到高达 131.7 mAh cm -2,实现了 28.2 小时的放电持续时间,估计原材料成本为 7.02 美元 kWh -1。这些指标显示了这种未锁定的氯铝酸盐电池在未来低成本、长期电化学储能方面的巨大潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号